
All finished Drug products/Medicine should be identified by labelling, as required by the national legislation, bearing at least the following information:
- The name of the drug product;
- A list of the active ingredients (if applicable, with the International Non-proprietary Names (INNs)), showing the amount of each present, and a statement of the net contents, e.g. number of dosage units, mass or volume.
- The Batch number assigned by the manufacturer.
- The expiry date in an uncoded form.
- Any special storage conditions or handling precautions that may be necessary.
- The directions for use, and any warnings and precautions that may be necessary.
- The name and address of the manufacturer or the company or person responsible for placing the product on the market.
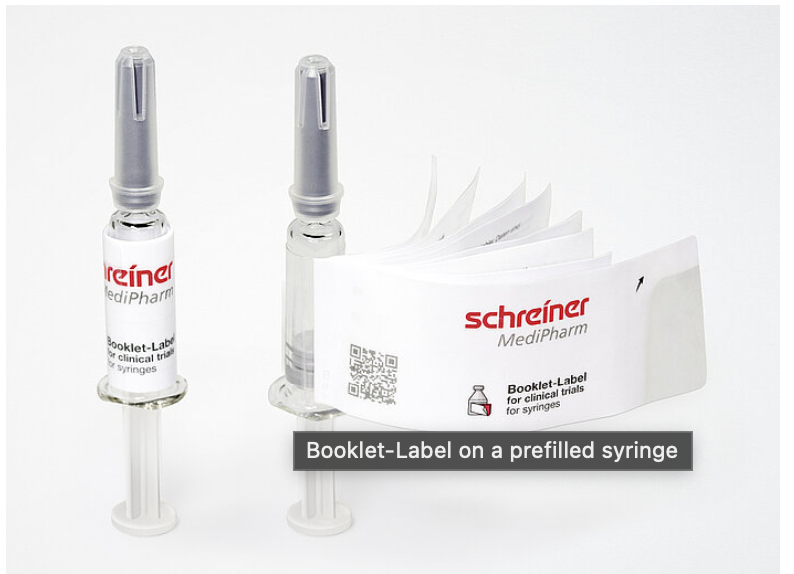
Special Note - Legal regulations require comprehensive information on pharmaceutical products. Frequently, this has to be available in multiple languages. Clinical trials, which are usually conducted internationally, also require detailed descriptions in the respective local language. With Pharma-Multi-Inform, Specialty labels for product marking that can save an extra package insert—as a multi-page booklet or multi-layer film label. All product variants offer sufficient space for multilingual information on the drug and its administration. The multi-page labels are developed specifically for each application and are suitable for primary or secondary packaging.
For any enquiry related to Functional Pharma Labels - email us at- info@packagingconnections.com or growth2@packagingconnections.com






