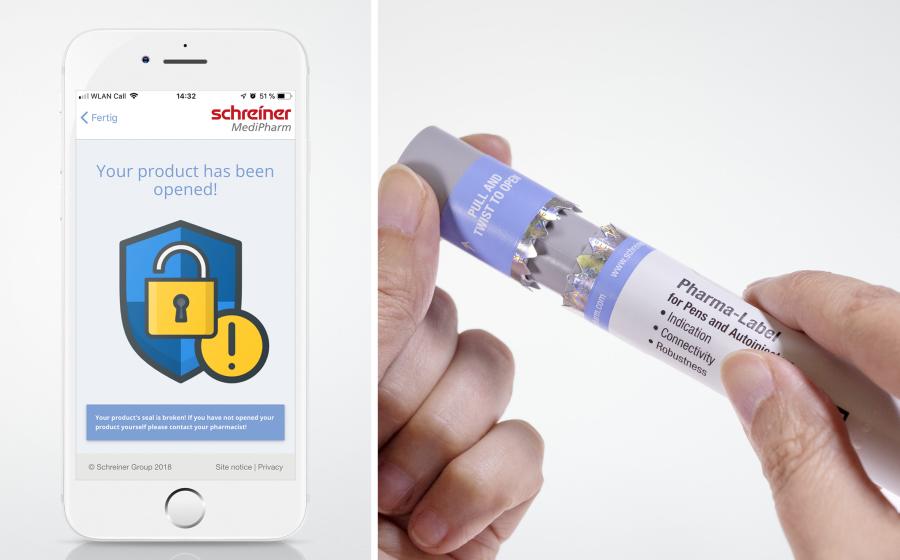
The EU Falsified Medicines Directive (FMD) requires pharmaceutical manufacturers to serialize their products and to provide secondary product packaging with an anti-tampering device. Primary containers or devices such as pens and autoinjectors for self-medication, however, are not included in these requirements yet. Schreiner MediPharm has developed an NFC-Label for autoinjectors that enables digital tamper evidence on primary packaging and makes it possible for pharmaceutical manufacturers to close this risk gap.
Schreiner MediPharm’s novel Autoinjector-Label wraps around the autoinjector including the cap and has an integrated NFC chip that can be read very easily via a smartphone app. Before opening the cap for the first time, the patient checks if the product is an original and receives respective confirmation. After opening the cap and label, and subsequently reading the NFC chip once more, a warning will appear on the smartphone that indicates whether the product was previously opened and may have been tampered with. Thus, patients are able to easily and quickly check the intactness of their injection aids anywhere, anytime.
Pharmaceutical manufacturers are able to integrate additional interactive applications in the label such as product information, demo videos or special apps to optimally support patients during self-medication. Additionally integrated geo-tracking makes it possible to detect potential gray market activities in local markets. The digital Autoinjector-Label for NFC-based tamper evidence can easily be adapted to existing label designs and does not affect the normal application of the device by the patient.
For pharmaceutical manufacturers, Schreiner MediPharm’s smart label solution enhances product and patient safety and also supports the integrity of the supply chain. At PDA Universe of Pre-filled Syringes and Injection Devices in Orlando/Florida in October, Schreiner MediPharm will present its new development to a professional audience for the first time.
