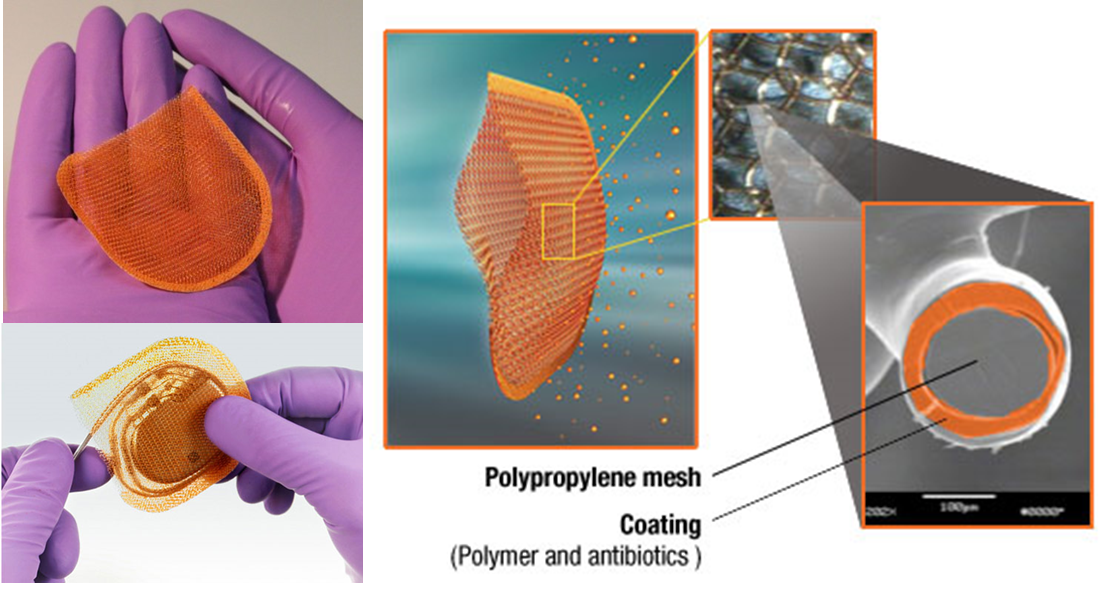
The AIGISRx R Antibacterial Envelope from TYRX, Inc. is FDA approved and a new fully resorbable “envelope” that encloses implantable cardiac devices, such as pacemakers and internal cardioverter defibrillators (ICDs), and helps prevent surgical site infections. AIGISRx is a dual component (resorbable and nonresorbable), sterile prosthesis constructed of open-pore weave, knitted filaments of a lightweight polypropylene mesh and coated with a resorbable polymer. The resorbable polymer contains the antimicrobial agents rifampin and minocycline, which are released locally into the tissue. The biocompatible mesh is coated with antibiotics that elute (dissolve) within a 7 to 10 day period. The resorbable polymer is resorbed by the body over the next approximately 140 days, leaving only the nonresorbable lightweight polypropylene mesh to hold and stabilize the device. The resorbable polymer is composed of nontoxic or naturally occurring materials. Each component of the polymer has a history of prior human use or human exposure as implants, IV injectables, or foods. Many of the polymer components are GRAS (generally regarded as safe). Once introduced into an aqueous environment, the polymer matrix absorbs water, beginning the process which drives degradation and elution.






