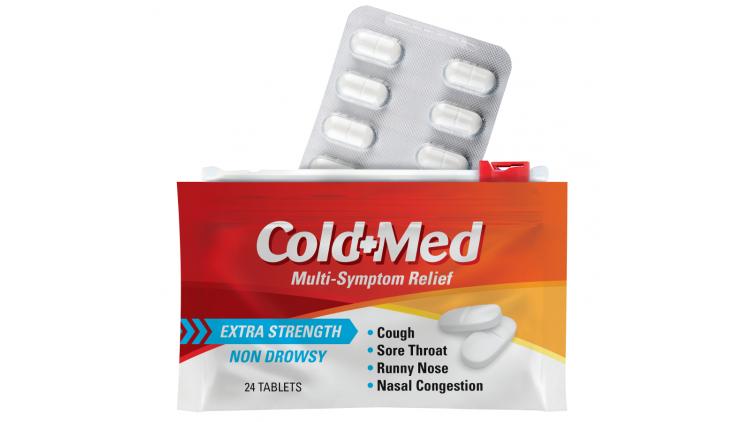
A uniquely functional pharmaceutical packaging concept, developed collaboratively by Bemis Healthcare Packaging and Presto Products Co., uses flexible packaging instead of paperboard cartons to protect blister packs.
The centerpiece of the packaging design is a pouch that provides both child-resistance (CR) and barrier protection for prescription and over-the-counter drugs. In addition, the design also offers merchandising flexibility for retailers and pharmacies.
The pouch features Presto’s Child-Guard CR track and slider, a closure designed to be difficult for kids to open but easy and intuitive for seniors.
According to Presto, the ergonomically designed closure has a Drug Master File listing, meaning it is FDA-compliant for use in pharmaceutical packaging. The reclosable slider meets the Poison Prevention Packaging Act’s CR requirements, as well.
The package also provides robust product protection. Bemis Healthcare Packaging converts the pouches using multilayer laminations that provide high oxygen and moisture barrier; depending on the product, the film may contain a layer of aluminum foil. Consequently, the primary blister pack can be made from cost-effective non-barrier materials—the pouch does the heavy lifting vis-à-vis product protection.
Multi-layer lamination of 92-gauge PET/35-ga foil/PE (PerfecPharm P510 is a three-ply structure with foil in the core. This lamination offers high moisture and oxygen barrier. The 92-ga PET is often used in foil laminations that will be used in CR packaging applications.
We also produced a pouch from a film/film lamination (PerfecPharm 35634-M is a two-ply structure). This non-foil lamination provides high puncture resistance suitable for CR packaging applications.Printing done by Either surface or reverse flexo print






