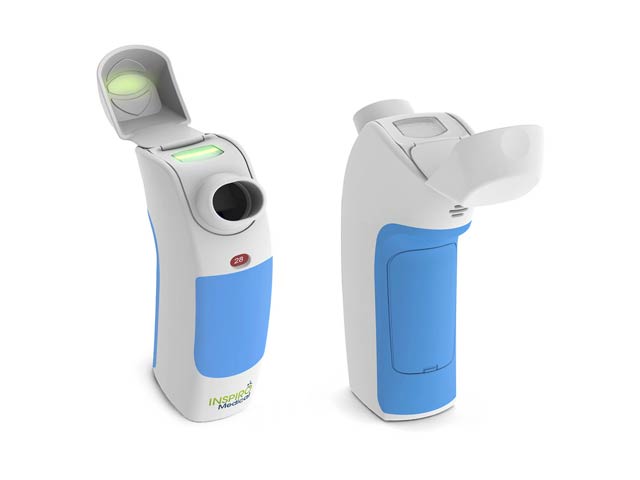
Inspiro Medical is developing Inspiromatic™, a breakthrough DPI (Dry Powder Inhaler) that uses advanced technology to overcome the drawbacks of current inhalers. inspiro’s proprietary DPI is a device to combine an active dry powder fluidization mechanism that works at low inhalation flow rates with a unique real-time feedback control and data logger indicating proper inhalation technique and follow-up. Inspiromatic assures optimal drug delivery. Young children and the elderly will particularly benefit from the advantages of Inspiro’s dry powder inhalation. In an asthmatic child , it is difficult it administer inhaled medications properly. Even if you can persuade the kid to hold still, you can’t make sure child is breathing deeply enough to get the treatment delivered effectively to the lungs. Dry powder must be inhaled forcefully to empty the premeasured capsule and to break down the drug particles enough to reach deep into the lungs. While many patients don’t use a DPI correctly, many others stop using it when they feel better – ignoring the need for maintenance. Physicians have no way of monitoring either of these situations, so their treatment decisions necessarily involve guesswork. Kaufmann’s invention addresses that problem too.
This smart dry-powder inhaler consists of three main features to overcome those issues.
- Inspiromatic’s active internal mechanism breaks down the particles via a flow sensor that detects the right time to deliver the medication and disperses the particles in the right size without need for forceful inhalation.
- If the patient inhales effectively, a green light comes on. If not, a red light comes on. A beep sounds when the unit detects that the whole dose has been delivered.
- The flow sensor and the internal microcontroller also store data in the device. It knows if the patient really inhaled correctly or didn’t, so the physician can access this data and see if the drug was delivered successfully and if not, why not. This makes it much easier to find the problems and provide solutions.
A working prototype, developed with the help of subcontractors specializing in various relevant fields, was tested in the lab using existing drug formulations. According to the Kaufmann “We had really good results over existing devices on the market,. Now we are also looking to raise $1 million to develop our device to the point where we can get the [European] CE Mark, but we still will need approval for the drug-device combination.” The long-term goal is to get approval in both the European and American markets.







