My Content
-
-
Eye dropper system with retained te-ring
-
Aptar's beauty & home division's 5 flags...
When we look for good and robust product dispensers and associated formats, the five letter word Aptar pops up immediately in our mind. Aptar is a leading global supplier of a broad range of innovative dispensing, sealing and active packaging solutions for the beauty, personal care, home care, prescription drug, consumer health care, injectables, food and beverage markets.
Aptar’s mantra is ‘Think Local, Leverage Global’. Keeping the same in mind, Aptar’s Beauty & Home India division, under the leadership of Kanwal and Supriya - the well known names in the packaging solutions provider industry - has rolled out five dispensers in the India and South Asia markets, namely Pacto, Micro, Alpine, Pearl4u and Click. Have you visited packagingconnections.com/aptar/ ? If not, then you are just one step away of exploring the much awaited exciting packaging formats in the dispensing category.
Pacto is a pocket friendly spray pump catering to the young and globally aware youth of Asia. The objective of launching Pacto was to design a dispenser that would connect with the youth and outperform in terms of aesthetics, functionality, convenience and performance. It is really value for money as one gets 350 sprays from a 25 ml pack with more details available on packagingconnections.com/aptar/pacto/
 Micro is an airless dispensing packaging format, the perfect solution for dispensing of products with model fill levels ranging from 20 to 50 ml. Airless technology in dispensing is a means to clean and neat 360º dispensing of the product. Negligible amount of product’s left over exists, providing value of money to the consumer. For more information, you can visit packagingconnections.com/aptar/micro/
Micro is an airless dispensing packaging format, the perfect solution for dispensing of products with model fill levels ranging from 20 to 50 ml. Airless technology in dispensing is a means to clean and neat 360º dispensing of the product. Negligible amount of product’s left over exists, providing value of money to the consumer. For more information, you can visit packagingconnections.com/aptar/micro/ Click is a double spring pre-compressed pump with exciting applications for which details are available at packagingconnections.com/aptar/click/
Click is a double spring pre-compressed pump with exciting applications for which details are available at packagingconnections.com/aptar/click/ Alpine is again a spray pump with applications in air care, deodorant, household and automotive segments. More information available on packagingconnections.com/aptar/alpine/
Alpine is again a spray pump with applications in air care, deodorant, household and automotive segments. More information available on packagingconnections.com/aptar/alpine/ Pearl4u is an innovative hair oil and serum dispenser which facilitates self application, saves time, reduces effort and solves the mess. Please visit packagingconnections.com/aptar/pearl4u/ for more details.
Pearl4u is an innovative hair oil and serum dispenser which facilitates self application, saves time, reduces effort and solves the mess. Please visit packagingconnections.com/aptar/pearl4u/ for more details.In case you want to know more on call, you can get in touch with us at +91 124 4965 784 / sales@packagingconnections.com
Your sample kit is waiting for you. Reach out ASAP.
Image
-
Abc's of package tests: stiffness (handl...
The Handle-O-Meter stifness tester has a flat plate to hold the specimen over a slot, a bar which forces the film through the slot and a strain gauge to sense the force exerted against the bar by the film sample.
After film sample is centered across the slot, the bar automatically descends to push the film through the slot against the resistance pf the film. The force necessary is sensed by the strain gauge an indicated on a calibrated meter dial. The maximum value of the force is taken as the Handle-O-Meter stiffness of the material.
Handle-O-Meter stiffness is reported in grams. Stiffness, whether it be reported as Handle-O-Meter or other means such as dynamic tensile modulus, is an important factor in machine operation, since often a material must be pushed and must not yield. Stiffness is the opposite of softness or limpness. Both properties find important application in sales appeal and package use.
Image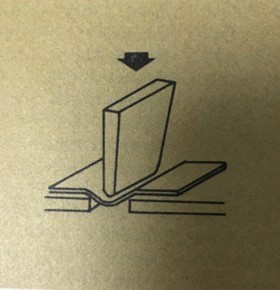
-
Freeze indicator: temperature monitoring...
About one in five temperature-sensitive pharmaceutical products is damaged due to a broken cold chain while being shipped. Particularly affected are sensitive substances such as biopharmaceuticals and vaccines. Therefore, seamless monitoring that indicates for instance if a pharmaceutical has been exposed to freezing temperatures is imperative. A label-integrated Freeze Indicator offered by Schreiner MediPharm in cooperation with American Thermal Instruments (ATI) provides a reliable and simple solution for monitoring cold chain compliance.
Further details at https://www.schreiner-group.com/fileadmin/sgr_forum/SGR_Forum_2019_03_EN/18/
The Freeze Indicator is based on ATI’s printed BlindSpotz™ technology, which Schreiner MediPharm integrates into labels for the pharmaceutical industry. Consequently, the application of these labels to vials or other containers enables reliable unit-level temperature monitoring. The functional principle is equally simple and effective: The Freeze Indicator is initially white. If the container with the specialty label is exposed to freezing temperatures below the defined threshold the color of the temperature indicator shifts to blue—unequivocally, readily visible and irreversibly.
Whereas previous solutions to indicate freezing temperatures are bulky, inflexible and cannot be modified in terms of design, Schreiner MediPharm’s Freeze Indicator offers utmost flexibility: The technology can be implemented using diverse printing techniques and easily integrated into existing label designs. In addition, it enables customized designs and is more cost-efficient than previous solutions. The label dispensing process in the pharmaceutical manufacturer’s operations remains unchanged. The greatest advantage of this technology, however, results from its form of application: Conventional temperature monitoring solutions are typically attached to secondary packaging. Due to the label application at unit level, the individual containers that have been exposed to freezing temperatures can easily be detected by means of the color shift, which effectively prevents the use of damaged medicines.
The Freeze Indicator benefits pharmaceutical manufacturers, healthcare staff and patients alike because potential damage to a medicine due to freezing temperatures is detectable at first glance. This supports a reliable supply chain and enhances patient safety. The label-integrated Freeze Indicator turns blue when a medicine has been exposed to excessive freezing temperatures and enables flexible designs.About American Thermal Instruments (ATI) Founded in 1981, American Thermal Instruments produces custom temperature monitoring solutions for industries that require the most accurate and measurable systems – pharmaceutical, medical, food, beverage, and Industrial.
Image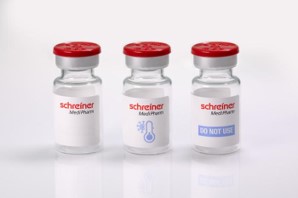
-
abc's of package tests: tear
A tear tester has a stationary clamp and a movable clamp on a pendulum, means for holding this pendulum in a raised position, then quickly releasing it, and a scale that registers the arc through which the released pendulum swings.
Samples of paper or fil are clamped into the tester and nicked to start the tear; then the pendulum clamp is released. This tears the sample and the scale registers the arc, As the arc i sproportional to the tear strength of sample, calibration of the arc gives the tear strength.
Tear strength is reported in grams/mm of thickness. It is the force necessary to continue tearing a sample after a nick has been made. This test is very importnat for all films as well as paper. High tear values may be needed for machine operations or for package strength. However, low tear values are necessary and useful for easy opening of some package types.
Image
-
Flexible packaging for two products
Two products with spouts in a single pouch is the great innovation patmented by Oleksandr Grytsenko from Ukarine. More details at https://duopack.org/patents-on-flexible-packaging-for-two-products
The essence of the industrial design is characterized by the decision on the original form and construction of the invention "Pouches for packaging, storage, transportation, distribution and use of liquid, gel, paste and other viscous products," which allows simultaneous or successive withdrawal of products from two different hermetically sealed tanks through two separate holes by mechanical action on the industrial design itself. This construction allows the consumer to use this industrial design in a combined form instead of several separate products in different pouches. Thus, the connection between the appearance of an industrial design and its construction gives it an individual character when compared with other existing industrial designs.
Image
-
Abc's of package tests : impact strength
The pendulum impact tester can be used to measure impact strength of films. An impacting head on the end of pendulum is swung through an arc into and through sample. Tester has a means of measuring difference between potential energy of pendulum at maximum height in free swing and potential energy of the pendulum after rupture of sample. This difference in energy is defind as impact strength and is reported in units of kilogram-centimeters. It is useful in predicting resistance of a material to breakage from dropping or other quick blows.
A test similar in scope, method and sognificance is the dart test for polyethylene film. Weighed dart is dropped from standard height onto taut sample. Significance and purpose are the same as in the pendulum test. Dart unit is weight of dart in grams that breaks sample 50 percent of the time.
These tests give an index of material's dynamic dtrength and approximate what will occur when package is dropped.
Image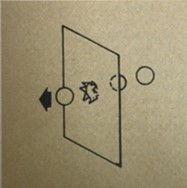
-
Abc's of package tests : tensile and elo...
The testing machine consists of clamps to hold the sample, some means of gradually increasing the load on the specimen until it breaks and indicators which show the load and the amount of elongation.
To perform the test, measured, gauged speciments are clamped into the testing machine and streteched until the break. Tensile strenth is usually reported in Kilo Newton per meter necessary to pull the papaer spart. For films, the usual units are kgs per sqcm of original cross-sectional area. Tensile strength is quite literally the amount of force necessary to pull a material apart. The elongation is the amount a material will stretch before breaking.
Tensile strength is a most important value for materials used in applications such as heavy-duty bags. A large value for elongation is an index of toughness, since it indicates a material will absorb a large amount of energy before breaking.
Image
-
Yato dharmastato jaya (sanskrit: यत?...
Yato Dharmastato Jaya (Sanskrit: यतो धर्मस्ततो जयः) a Sanskrit shloka. and it means that "Where there is adherence to right action, there [alone] lies victory!" Same is true in business ethics to be successful.
Some people say we got to be practical as they come with the arguments that an omelette can not be made without breaking eggs. But this could be true for omelettes but not for the victory. We strongly believe that this also holds true for the packaging of various products. If we overpack any product with extra packaging then it doesn't really help and has an adverse impact on our environment. So if we simply relook at various packaging for so many products, I am sure a lot could be reduced.
For example, we see big blisters for small memory cards or so many electronic items. Removing this big blisters can would lead to bi elimination of packaging.
So there is no need to put extra packaging just to increase the packaging business, there are enough products which need packaging solutions to protect themselves and we should then direct our energy towards those ignored products or ignored markets.
Image
-
The impact of packaging on the success o...
E-commerce has taken the world by storm. Its massive growth has provided a sea of opportunities for the packaging industry and its future prospects appear only brighter. Today a dissatisfied consumer can easily post his/her review on any online platform and it would not be an overstatement to say that a single review has the power to influence the purchasing decisions of other potential customers. Therefore it has become imperative to keep track of the changing trends in consumer preferences. Moving in this direction, Sanex Packaging Connections is making a sincere endeavour to study the impact o f packag ing on the success of e-commerce.
Our team started a survey on 31st May 2019 to study the impact of packaging on the success of e-commerce. We have received overwhelming response on the survey. To make our analysis and report more detailed and realistic and to hear more from our readers, we have extended the survey closing date from July end to 15th Aug’19. Shortly after that the analysis will be shared with you all.
Follow the given link to participate in the survey - https://www.surveymonkey.com/r/packaging4ecom
Image
-
Micro: airless packaging’ by aptar b+h...
Every packaging professional whether in Consumer Packaged Goods industry or in Pharmaceutical company craves for using airless dispensing of products such as creams and lotions for various obvious reasons.
First, airless technology in dispensing is a means to clean and neat 360º dispensing of the product. Second, negligible amount of product’s left over exists, providing value of money to the end user, the consumer. Third, airless dispensing reduces the contamination of the product with the particulates in the surrounding environment to the minimum, thus ensuring safe usage. Finally, it’s an amazing technology with many! many! many advantages.
We all know that the Aptar is the Global leader in the world of dispensing. Aptar India’s Beauty & Home vertical has come out with an airless dispensing packaging format termed as ‘Micro’, the perfect solution for dispensing of products with model fill levels ranging from 20 ml to 50 ml.
For more information, please feel free to write to us at info@packagingconnections.com. Parallely, get your sample at the earliest by filling the details in the Product Enquiry section of the web page with URL as https://www.packagingconnections.com/aptar/micro/.
-
Importance of appropriate pharma packagi...
Undisputably, the eyes are one of the most important and highly sensitive organs in the human body. The slightest irritation in the eyes can cause extreme discomfort and lead to eye infections, swelling, allergies, challenge vision and may lead to permanent damage and cause loss of vision as well.
This is also the reason why Sterile and correct packaging of pharmaceutical products pertaining to ophthalmology is extremely crucial. We can further understand why the pharmaceutical packaging of ophthalmic products cannot have a window or possibility of an error during packaging, shipment and delivery.
What are some common Ophthalmic products?
Ophthalmic products are sterile preparations which include specialized dosage forms that are administered onto the external surface of the eye (inside or adjacent to the eye), contact lenses, specialized surgical implants, eye gels, lotions, powders, parenteral products, ophthalmic inserts, prefilled syringes, etc. All Ophthalmic products are required to stringently comply with Test for sterility as per the pharmaceutical manufacturing and packaging standards.
Different pharmaceutical companies employ various methods and materials to package Ophthalmic products. The most commonly used materials used for packaging of Ophthalmic products are plastic bottles which are generally made of Low-Density Polyethylene (LDP), Polypropylene (PP), High-Density Polyethylene (HDPE) etc depending on the specific product requirement.
Apart from plastic, traditional Boro-silicate (Type 1 glass) glass bottles with the rubber dropper which were commonly used until the 2000s but have now been replaced by Low-Density Polyethylene (LDPE) bottles.
Eye ointments continue to be packaged in Flexible plastic or Collapsable Metal Tubes.
In order to prevent contamination and leakage of product, Caps and Closures, nozzles etc are made using Polypropylene (PP). The Caps and Closure for Ophthalmic products are also Colour-Coded depending on their Pharmaceutical Class.
Importance of Correct Packaging of Parenteral Ophthalmic Products
Parenteral products are administered directly into the body tissue through the skin and the mucous membranes. It is also crucial that these products need to packaged with extreme care to ensure that the product is delivered with exceptional purity and free from all physical, chemical and biological contamination. The pharmaceutical packaging material used for ophthalmic products should not adversely affect the quality of preparation or allow any diffusion and the packaging must be insusceptible to leakage, contamination etc; besides including a tamper-evident seal.
The Pharmaceutical Packaging industry calls for stringent adherence to current good manufacturing practices (cGMPs) to ensure the packaging on delivery of ophthalmic products.
Several innovative and new pharmaceutical packaging formats are being developed every day to meet the growing requirement for sterile, safe and secure packaging and delivery of ophthalmic products across the world.
Apart from the packaging and delivery of ophthalmic products, the important information pertaining to the dosage, administration, product details, ingredients, storage, date of manufacture, date of expiry, manufacture details and other crucial information related to the product is found printed on the actual ophthalmic product. The vital product information found on the pharmaceutical packaging of ophthalmic products is also extremely important for pharmacists, doctors, patients and other health care professionals who are responsible for dispensing and administration of the ophthalmic product.
Image
-
Impact of packaging on the success of ec...
Survey link https://www.surveymonkey.com/r/packaging4ecom
E-commerce has taken the world by storm. E-commerce markets are growing at staggering rates. The online market is expected to grow by 56% in 2015–2020. In 2017, retail e-commerce sales worldwide amounted to 2.3 trillion US dollars and e-retail revenues are expected to grow to 4.88 trillion US dollars in 2021. Traditional markets are only expected to have 2% growth during the same time. On the other hand, brick and mortar retailers are struggling because of online retailer's ability to offer lower prices and higher efficiency. Its massive growth has provided a sea of opportunities for the packaging industry and its prospects appear only brighter.
Today a dissatisfied consumer can easily post his/her review on any online platform and it would not be an overstatement to say that a single review has the power to influence the purchasing decisions of other potential customers. Consumers can also share experiences, videos and images of their product on social media and when they do that the spotlight is not only on the product but also on the packaging. Getting an insight into a consumer's likes, dislikes, ideas and expectation has become crucial.
Therefore, it has become imperative to keep track of the changing trends in consumer preferences. Moving in this direction, Sanex Packaging Connections is making a sincere endeavor to study the impact of packaging on the success of e-commerce. We aim to decode this intimate relationship between e-commerce and packaging and through that exploit the unutilized opportunities.
We request you take out few minutes from your busy schedule to complete our survey. We assure you that your responses will remain confidential and will be used only for research purposes.
Please follow the link to participate in the survey: https://www.surveymonkey.com/r/packaging4ecom
Image
-
Impact of packaging on pharma products: ...
Pharmaceutical packaging is a necessary component for Ophthalmic products. It guarantees the stability and integrity of the products and represents the pharmaceutical brand itself. It also provides critical information to patients and medical professionals during drug delivery.
Pharma packaging must be in accordance with drug compatibility and patient compliance. The information must be clear, as to advise patients and medical professionals when no other source of information is available. Its not just the label claim but interaction between the drug & packaging material, shelf life, functionality and regulatory clearance.
In addition to the labeling and leaflets provided, proper packaging guarantees accurate use of medicinal products. This promotes compliance and safe administration, as in the case of blister packs indicating which medications to take at what time. Safety is another component of proper pharma packaging reducing the risk for children in the way of safety caps.
Most importantly, pharma packaging helps users identify correct drugs. Confusion with inadequate pharma packaging can lead to serious consequences for patients as well as medical professionals.
The impact of packaging on pharma products is great and warrants serious consideration when choosing the right pharmaceutical products for Ophthalmic products.
Ideal Packaging for Pharma Products
 Containers should be tested for resistance against various elements, such as chemicals and water. Proper packaging for pharmaceuticals preserves the therapeutic effectiveness of medicines until consumed. This includes testing done during the packaging process, storage, transportation, and also dispensing.
Containers should be tested for resistance against various elements, such as chemicals and water. Proper packaging for pharmaceuticals preserves the therapeutic effectiveness of medicines until consumed. This includes testing done during the packaging process, storage, transportation, and also dispensing.Another critical factor is the interaction between printed label & the container. Many a times some unwanted ingredients may migrate to drug from packaging like Benzophenone. So the study of extractable & leachable is critical even though the label on the container is secondary packaging but plastics are porous in general.
The factors that are most important to consider in ideal packaging for pharma products are:
· Medicinal products should be safe from leaking or spoiling.
· They must be protected against moisture, light, and other elements that could impact the integrity of the medicinal product.
· Resistant to transportation demands.
Each medicinal product requires its own type of packaging to ensure ideal preservation. Consideration involved in selecting the right container is chosen according to:
· The shape and amount of the medicinal product determines the shape and size of the container.
· Container must be easy to open for patients, but hard to open for children.
· Container must resist water and moisture to preserve the integrity of the product.
The amount of medicine being dispensed at one time can also determine type of container. For instance, blister packs improve patient compliance as only a certain amount of medicine can be dispensed at one time, while with a bottle, a number of medicines can be taken at one time.
Many pharmaceutical companies have improved their compliance packaging designs to improve adherence. For example, in one design as doses are taken, perforated tabs can be removed, so patients take the correct next dose. While many people use pill containers, this solution isn’t as effective as with pharma packaging that comes pre-scheduled. Patients often fill pill containers incorrectly, which can lead to missed doses and worse, overdoses. In addition, removing pills from containers to place in pill reminder containers expose the medications to toxins and environmental elements affecting the integrity of the products.
Pharmaceutical Packaging Solutions

The pharmaceutical packaging industry has grown significantly over the last few years in attempts to provide adequate solutions for medicinal products. Many of the pharma packaging systems designed in the past do not adhere to the terms and conditions set forth by various regulatory authorities but now you can get packaging components with regulatory compliance. Reviewing current pharma packaging solutions is an integral part of any successful pharmaceutical company. Contact us today for more information about how we can help improve pharma packaging for Ophthalmic products. We offer packaging consulting services at www.PackagingConnections.com
Image
-
Pocket friendly perfume
8th April 2019 - Product focus - PACTO
What comes first in our mind when we listen this word PERFUME? “Fragrance”. And when we give a thought about its packaging, we imagine a classy glass bottle with an innovative shape, design, cap and what not….
But when it comes to carry it while travelling, well, we may end up thinking number of ways to carry this glass bottle and save it from any damages or we may even end up not carrying it at all.
Ever thought of carrying your perfume with you in your pocket for using it anytime and anywhere? Yes, it is possible with Aptar’s new product range - PACTO
This new packaging format is of convenient size to fit anywhere in small spaces. This comes in varied colours to compliment the style, is free from any mess, no dripping and overall gives a smart look and appeal to your product. Net sprays in 25ml product is equivalent to 350 sprays, lasts longer, hence, gives value for money.
Not only for perfume, this technology can be implemented for diverse range of products such as facial sprays, toner, cosmetic spray, makeup remover, automotive (freshness sprays), room freshener, deodorant, and similar products.
Aptar supplies this product as an assembly of three components:
1. Bottle + Shoulder cap assembly (1)
2. Pump module with tube
3. Actuator assembly (blue color)So, this product PACTO complements to the subject - Pocket Friendly. First, it is a small sku that means less cost (pocket friendly) than that of bigger variants. Second, it fits into your pocket perfectly.
For more information about this product, contact our team here: https://www.packagingconnections.com/aptar/pacto/
Image
-
Benchmarking in packaging
By definition –
- Benchmarking is an improvement process used to discover and incorporate best practise into operation.
- It is a process of identifying, understanding and adapting outstanding practices from organization anywhere in the world to help tour organization improve its performance
- It is a highly respected practice in the business world
Specific to packaging, Benchmarking determines –
- Which is the best packaging in terms of appeal, material selection, space utilization, consumer convenience and so on?
- How effectively the product is being packed?
- How is the competitive brand doing that?
- Where does our brand stand in competition?
- What are the areas where our brand can be optimized?
- What are the cost breakups of the packaging involved?
- If we are already optimized, is there a scope to look for some new innovations?
- Identifying new vendors, new materials, new systems in packaging
Benchmarking aims at INNOVATING and not IMITATING
Testing a package can be done inhouse or by outsources laboratories. However, the later doesn’t analyse the data, they only present it in a codified manner. To analyse the data either a packaging professional had to dedicate his/her time else they simply use the data and present. We at Packaging Connections provides “Value Additive Benchmarking” across various companies and product categories.
Our detailed analysis include –
- Benchmarking for competition companies/brands/locations/products and even cross category also.
- Tear Down Analysis of the product (Rigid / Flexible packaging)
- Technical data in a detailed matrix of different parameters
- Inhouse test + outsourced testing
- Visual, Analytical, Calculated and Measurable parameters – all covered
- Data analysis and identification of improvement areas
- Cost analysis and cost breakup of various packaging formats
- Innovative ideas of the related product packaging and cross categories
- Detailed pdf report to follow along with parameters analysed
- Present the data in a “ready to use” format for quick implementation and presentation by any packaging professional
- Post project support for any discussions on technical inputs being provided
For more information emails us at – info@packagingconnections.com
-
Recipe for success by #shivkhera
The key message here is that few positive actions must be repeated and that too daily. Repetition is the mother of learning, it brings consistency. Consistency results in proficiency, it is proficiency that shapes our lives. Afterall consistency signifies credibility.
Same is true for the success in packaging, since packaging function is largely a service function so we must make sure that our external or internal customers are well taken care, and to do that we have to formulate few actions which must be repeated daily, for example sharing packaging news
Image
-
Why no to plastics?
But why can’t NGT or various municipalities work kn creating collection and recycling infrastructure, simply banning is the band aid solution but not the permanent solution, in fact it leads to more corruption. It is also spoiling #swachhbharat mission and I wonder what is national premier packaging institute doing?
I think we need to form a sincere NGO by packaging and plastics professionals to address this issue. Developed nations like Europe, US use much more plastics per capita than we developing/emerging nations but we have all the problems, the real issue is we only look at one side of doing good business but not in totality.
The premier national packaging and plastics institute who are not sincere to resolve such issues should be questioned and have to be accountable. Plastics institures can not simpy teach plastics and run away or packaging institutes simply tell how to use but nothing more on disposable should also be accontable. The sustainability has to be part of curriculum
Image
-
Wind of change : the future is in the ai...

Do you know the song Listen to Wind of Change? It’s the song by the group Scorpio formed in Germany. This was written in 1989 and the album was published in 1990. This happened when Berlin wall came down, communism went away from USSR etc. So many changes and so the group was highly impressed and wrote this song. We at PackagingConnections.com are quite impressed by this song and if you do not know, please listen here:
https://www.youtube.com/watch?v=n4RjJKxsamQ
There is also a Chinese Wisdom:
If the wind of change is blowing, some build the protection walls, others build windmills.
So here is our message that changes are happening at www.PackagingConnections.com and you would have already started noticing them if not then you will soon see them. We are buliding packaging windmill called www.PackagingConnections.com to share the latent power of packaging with all our packaging community. We beleive that this latent power of packaging would benefit humans to protect products and improve the life. You are most welcome to distribute further our newsletters, send us your feedback anytime.The latest change if the Big Book of Packaging, for more details www.bigbookofpackaging.com
We would keep you updated with the changes happening here so that you can listen to the winds of change and enjoy. This is for you to enjoy.
Happy blowing the wind of change!
Sandeep Goyal
Founder & CEO
www.PackagingConnections.com



.png)






