Serialization: a pharmaceutical packagin...
Serialization is the provision of a unique serial number to each saleable unit of a product. This number provides information about the product’s origin, manufacturing, and expiration date and works as an identifier for tracking. Serialization codes are generated either randomly or sequentially.
Serialization is important (and obligatory in most countries), especially in the pharmaceutical industry, as it protects against counterfeit drugs.
Different countries have different regulations for serialization although global GS1 standards are also considered. GS1 is a non -profit organization that develops and maintains global standards for business communication-based in Belgium.

The following elements are required to complete serialization, according to GS1:
GLN and GTIN: GLN stands for the global address of operation and GTIN is the unique complement of the manufacturer’s products around the world.
Serial Number: Numeric or alphanumeric character sequence consisting of up to 20 digits which must be unique for each GTIN.
Lot Information: The same as Batch Number, used to determine the production cycle of the company and expiration date.
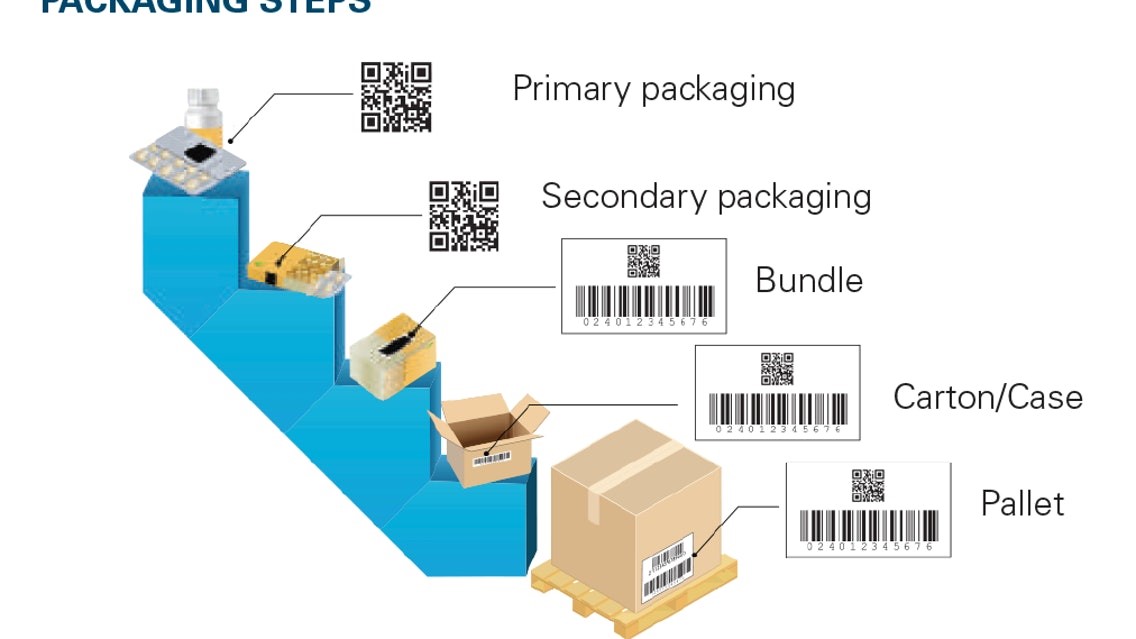
In India, the law requires manufacturers to uniquely serialize drug products at the tertiary (case), secondary (saleable unit), and mono-carton packaging levels. Different packaging levels also have different requirements in terms of the data being presented. For instance, at the secondary pack level, the GTIN, batch number, expiration date, and serial number must be encoded in a 2D data matrix or at GS1-128 linear barcode.

As technology progress and regulations become more strict, there will be a shift in packaging design to make packaging fully traceable throughout the distribution chain.  Data management capabilities will also grow rapidly in order to keep up with the need for excessive data collection, processing, and storage.
Data management capabilities will also grow rapidly in order to keep up with the need for excessive data collection, processing, and storage.












 Now been in use for decades, blister packaging is the go-to option for pills and tablets. The visual evidence of a tear on the foil seal is usually enough to indicate to the consumer that the contents have been compromised.
Now been in use for decades, blister packaging is the go-to option for pills and tablets. The visual evidence of a tear on the foil seal is usually enough to indicate to the consumer that the contents have been compromised.