Activity
-
Stefano Rossi, CEO of packaging at DS Smith said, “We were approached by several of our food supply customers to design
-
Aipia world congress, the only global sm...
Live Broadcasts, Brand Challenges, Technology Streams, One-to-One chats and Virtual Booths.... Following extensive consultation with the industry AIPIA, the Active & Intelligent Packaging Industry Association has decided that the 2020 World Congress will be run as a Virtual event, returning as a physical Congress in 2021. The date for AIPIA VC is September 10, 2020 Making the announcement AIPIA’s managing director Eef de Ferrante said, “We spoke to the industry’s leading players across every product and service and it was absolutely clear this was the right decision at this time. The world may soon be recovering from the COVID-19 pandemic, but the effects on business activity will go on for some time. So we decided the Congress is just too important to be spoilt by these disruptions.” “We have carefully researched various Virtual event technologies in a very short space of time and come up with a system which allows us to bring all the things you have come to expect from our Congress. So it will include live broadcast presentations, online booths, live Q&A with speakers, one-to-one chats with technology providers and a real Online Challenge,” he explained. The Congress is, and continues to be, the only truly global event covering the entire sector. It is simply the most important place for networking and information exchange in the world of Smart Packaging. No other event allows delegates to see the Big Picture and meet so many Brands. This will continue in the Virtual event, so we come back even stronger, physically, in Amsterdam, 2021. “Our Advisory Board, which l am privileged to lead, were absolutely clear that the AIPIA Congress is just too important, and must happen in a way which allows it to continue helping to shape the future direction of the industry we all belong to. AIPIA is integral to the future success of the Smart Packaging sector and l urge you to give it your full and absolute support in 2020. The Virtual Congress must come first in your agenda,” stated Dick de Koning, Chairman of AIPIA. “This is a challenging time, but also an exciting one,” de Ferrante continued. “Many events have been cancelled or postponed and others are going to take the virtual route, so it will get crowded. But, l assure you, AIPIA Virtual Congress will be the best on offer for Smart Packaging practitioners and users. And it has the potential to reach a bigger ‘on line’ audience than ever, to add even more value for speakers, exhibitors and delegates. So making the choice on where to spend your budget is simple, we hope! ” he concluded.Image
-
Aptar requests u.s. fda emergency use au...
Aptar, a global leader in drug delivery, consumer product dispensing and active packaging solutions, is seeking U.S. -
Recycled content guidelines in plastic p...
THE British Plastics Federation (BPF) has teamed up with the Cosmetic, Toiletry and Perfumery Association (CTPA) and the -
Glenroy inc. announced as best of catego...
MENOMONEE FALLS, Wis., April 23, 2020 (GLOBE NEWSWIRE) -- Glenroy™ Inc., a converter and printer of flexible packaging for over 50 years, received two (2) awards in the Great Lakes Graphics Association (GLGA)’s 2020 Graphics Excellence Awards competition. The judging was conducted on March 10-11, 2020, by a team of independent judges. The GEA competition is one of the largest printing competitions in the nation. Glenroy won the Best of Category Award in the category of “Flexo-Wide Web, Process” for Original Superfood Creamer® and the Award of Excellence in the category of “Flexo-Wide Web, Process” for Unsweetened Superfood Creamer®. According to Mark Acree, Glenroy’s printing manager, “We have a great team at Glenroy that’s focused on quality and efficiency. We have something to really be proud of considering the competition from many package printing companies during this year’s Graphics Excellence Awards.” A panel of out-of-state judges with extensive experience in printing and print production was brought in to examine the work. Each entry was judged on its own merit in a category with similar printed pieces. The judging criteria included registration, crossovers, clarity and neatness, sharpness of halftones and line drawings, richness and tonal qualities of color, paper and ink selection, ink coverage, difficulty of printing, effective contrast or softness, finishing, bindery, and overall visual impact. About Glenroy Inc. Since 1965, Glenroy Inc. has been a trusted converter and printer of sustainable flexible packaging. A privately-held company headquartered in suburban Milwaukee, Glenroy manufactures high-quality flexible packaging films and stand-up pouches for a variety of end uses, including food and beverage, household and personal care, pharmaceutical, nutritional, cosmetic, animal health and pet care, medical device and industrial. Glenroy is also the exclusive converter of the premade STANDCAP Pouch. For more information on Glenroy's flexible packaging solutions, visit www.glenroy.com or call (800) 824-1482. Editorial Contact Ken Brunnbauer 800-824-1482 ken.brunnbauer@glenroy.com www.glenroy.comImage
-
Mitsubishi hitec paper receives the seal...
Mitsubishi HiTec Paper Europe GmbH, with its Bielefeld and Flensburg locations, is the first company in the paper industry to receive the "Safe with a System" seal of approval from the BG RCI: the professional association for raw materials and the chemical industry. At the same time, the company has been certified for occupational health and safety management (“AGM”) in accordance with DIN ISO 45001:2018. The DIN ISO 45001 for occupational health and safety management "AGM" replaces the BS OHSAS 18001:2007 standard, which was withdrawn in 2018 and was mainly used by companies in the chemicals, paper and sugar sectors. The new standard now also requires the assessment of risks and opportunities and a proactive prevention approach, thus increasing the demands on senior and middle management. When companies are preparing for assessment for the "Safe with a System" seal of approval with the additional module DIN ISO 45001, certain legal requirements have to be met and competence criteria for employees, whose participation play an important role, have to be determined. Managing Director Dr. Martin Schreer is convinced that "If you think about the safety and health of your employees from the outset, you will achieve more for your company and your economic success in the long term. This assessment and the certification of our occupational health and safety management are logical steps for us to further advance occupational safety in our company. Our exemplary company integration management, which was awarded the "Action Alliance Schleswig-Holstein", ideally complements the “AGM” system." The new DIN ISO 45001 certification is another component of the voluntary compliance system at Mitsubishi HiTec Paper. At the same time, the monitoring audits of the integrated management systems DIN EN ISO 9001 (quality management), DIN EN ISO 14001 (environmental management) and DIN EN ISO 50001 (energy management) have been carried out successfully for both locations. Regular certifications by the independent auditors from TÜV Nord underscore the high demands that Mitsubishi HiTec Paper places on itself in order to continue to be a reliable partner for customers. Mitsubishi HiTec Paper Europe GmbH is a German subsidiary of Mitsubishi Paper Mills Ltd., Japan, one of the world's leading manufacturers of specialty paper. The roughly 750 employees at Mitsubishi HiTec Paper Europe produce high-quality direct thermal, inkjet, carbonless, label and barrier papers at two tradition-rich locations in Bielefeld and Flensburg. Each factory stands out for own base paper production, state-of-the-art production machinery and innovative coating technologies. Through its dense global sales network, Mitsubishi HiTec Paper Europe supplies a full range of specialty papers for many applications and printing technologies – and is a highly capable partner whenever customized coated paper solutions are required. www.mitsubishi-paper.com Bielefeld Mill | Mitsubishi HiTec Paper Contact: Ralf Buhl Marketing Manager Mitsubishi HiTec Paper Europe GmbH Niedernholz 23 | 33699 Bielefeld | Germany Tel. +49 (0)521 2091-582 ralf.buhl@mitsubishi-paper.com www.mitsubishi-paper.com Reprinting permitted free of charge, specimen copy requested. www.mitsubishi-paper.comImage
-
Aptar pharma is an outstanding issue spo...
“Is Now the Time to Shake Up the pMDI Environment?” by Chris Baron, Director Business Development, Rx, EMEA at Aptar Pha -
Aptar and sonmol partner to develop digi...
This collaboration will initially focus on bringing together connected drug delivery devices and the digital platform fo -
The 53rd manufacturing bean jam basic co...
This event was held for customers who purchased Kajiwara's confectionery machine. -
Kallfass verpackungsmaschinen gmbh honor...
Once again this year, the managing director Jens Kallfass was able to honor jubilees at the Christmas party. -
Foil packaging is perceived differently ...
The current situation clearly shows how important packaging in general and film packaging in particular are and not only -
Ideal for hard-bricked foods, easy-to-bu...
2.Production time reduction and quality improvement brought by α mechanism Production time is greatly reduced, quality i -
clean blue air with dmsterile
Germ-free and virus-free mould dehumidification with DMSterile Programme expansion for aseptic production Kundl (Austria), 21.04.2020: For food and pharmaceutical manufacturers, sterilising the production areas is a common practice. When producing packaging for the same manufacturers, there is additional demand for sterile and low-particle plastic production. Blue Air Systems has developed a new product for this sector: the proven DMS (Dry Mould System) dehumidifiers are now available in the germ and virus-free version, DMSterile. DMSterile directly generates a germ and virus-free atmosphere during mould dehumidification. The end products, such as pharmaceutical containers, PET pre-forms or sealing caps, come into contact exclusively with sterile air during production within the partitioning. Advantages of germ and virus-free production Micro-organisms thrive where moisture and heat are present. Both conditions are often found in production halls. In addition, outdated or irregularly maintained filters of air conditioning systems, ventilation systems and even production machines facilitate the multiplication of germs and viruses. Aseptic production with DMSterile ensures an optimum environment for manufacturing plastic products and avoids costly after-care. Aseptic dehumidification with Clean Blue Air Aseptic dehumidification with the Clean Blue Air system provides sterile air quality without any micro-organisms in the production process. Together with the existing dehumidification technology, DMSterile improves the quality of the end product. With DMS dehumidification, the energy requirement of the production process is reduced by up to 80% due to the known energy savings, while increasing the quality level and output. The manufacturer Blue Air Systems provides further information and the opportunity to make a configuration query based on the desired process air volumes at clean@blue-air.at. Contact Blue Air Systems GmbH Achenfeldweg 8 A-6250 Kundl/Tyrol Austria Tel.: +43 (0) 5338 211 71-0 Fax: +43 (0) 5338 211 71-20 Email: info@blue-air.at Website: www.blue-air.at Managing Director: Bernhard Stipsits Blue Air Systems at a glance Blue Air Systems GmbH, founded in 2010 and based in Kundl/Tyrol (Austria), supplies the plastics processing industry with innovative technology for energy saving. Blue Air Systems, the leading innovator, has over 30 years of experience with effective, air-based process solutions for plastics technology. In the core field of air-conditioning technology, Blue Air Systems stands for solutions with cryogenic or dry air for energy-efficient processing for the plastics industry and other industries. Blue Air Systems develops not only high-quality, but above all safe and easy-to-use solutions that require as little energy and maintenance as possible. The company's product-supporting services include application and service-oriented advice for processors. The main products and applications in air conditioning technology include systems for mould cavity dehumidification (BAS-MSP and BAS-DMS series) and internal product cooling (BAS-CAC series) for effective cooling of blow-moulded parts using extremely cold and dry compressed air (-35ºC) (process volumes 120 to 540 Nm³/h). BAS-CAC significantly reduces material stress and cooling times by up to 50%. In the field of material handling, Blue Air Systems offers a comprehensive range of compressed air-based resin dryers for efficient and gentle material processing (RDM, RDX and RDL series) based on the Venturi principle. The use of the compressed air available from the company is a very economical alternative to conventional drying systems, such as adsorption dryers. The compressed air technology with process volumes from 0.5 to 1,000 litres guarantees the best drying results - grain by grain, with minimum operating costs and virtually maintenance-free production. A worldwide network of sales and service centres through representatives ensures optimum support for users and long-term value retention of the solutions used. Constant growth, references in more than 35 countries worldwide, innovative technologies and a high quality make Blue Air Systems a globally established and reliable partner for the plastics industry. Blue Air Systems has 15 employees (2020) and an export rate of about 98%.Image
-
Paper packaging for a sustainable future
Research and development is very important at Sappi, the result of which is the development of an exceptional level of e -
Foba supports manufacturers with webcast...
FOBA supports manufacturers with webcasts and flexible marking solutions during the crisis Fast provision, uncomplicated financing models and extended service make it easier to cope with production peaks In FOBA’s webinar series, laser marking experts offer direct part marking backgrounds and practical application knowledge Laser marking ensures safe traceability as well as counterfeit protection Selmsdorf, April 2020 – FOBA Laser Marking + Engraving (Alltec GmbH) offers support for manufacturers that need to cope with special requirements in the Corona crisis. The company, which is a leader in the market for industrial part marking and engraving with laser technology, provides flexible investment models for a quick and easy deployment of additional marking systems.
 “We are aware that many of our customers are currently struggling with declining orders, but at the same time see the increasing demand in certain areas such as the medical industry. We recognize this social responsibility and want to help as best we can,” says Managing Director Stefan Heczko. This applies particularly to medical device manufacturers whose production is ramping up or who are changing their portfolio due to current demands, which often results in an increased need for direct part marking.
“We are aware that many of our customers are currently struggling with declining orders, but at the same time see the increasing demand in certain areas such as the medical industry. We recognize this social responsibility and want to help as best we can,” says Managing Director Stefan Heczko. This applies particularly to medical device manufacturers whose production is ramping up or who are changing their portfolio due to current demands, which often results in an increased need for direct part marking.FOBA responds to this with economical and uncomplicated loan and leasing offers as well as extensive remote service, online training and webinars, but also on-site installation help. For the first time FOBA is offering short-term machine rentals for medical device manufacturers. Depending on the actual requirements, appointed rental or leasing models can also be extended to purchase, which gives maximum flexibility in terms of machine availability. The offer applies to FOBA's closed marking workstations or to marking systems that are integrated into production lines.
In order to use the time in the home office as profitably as possible, FOBA offers a series of webinars in April, May and June. Customers and interested parties will then also receive detailed information on laser marking with a focus on UDI labeling of medical devices in accordance with the requirements of the MDR. More information about FOBA’s webinars and regular updates can be found at https://www.fobalaser.com/webinar/
 FOBA's camera-based marking solutions are optionally offered as part of the M- Series closed marking workstations or of the safety class 4 marking systems for lineintegration. In this area, FOBA has recently launched innovative new solutions to the market, including FOBA TitusTM, the world's smallest marking head. This makes installation in the production line easy due to flexibility, simple assembly using clamp brackets and, above all, the extremely small space requirement.
FOBA's camera-based marking solutions are optionally offered as part of the M- Series closed marking workstations or of the safety class 4 marking systems for lineintegration. In this area, FOBA has recently launched innovative new solutions to the market, including FOBA TitusTM, the world's smallest marking head. This makes installation in the production line easy due to flexibility, simple assembly using clamp brackets and, above all, the extremely small space requirement. FOBA's laser marking systems keep pace with the legal requirements for traceability and create codes with high contrast and long durability, even on products that must not corrode and are subject to heavy use, e.g. through repeated sterilization. A capable marking software enables connection to all common industrial interfaces.
FOBA's laser marking systems keep pace with the legal requirements for traceability and create codes with high contrast and long durability, even on products that must not corrode and are subject to heavy use, e.g. through repeated sterilization. A capable marking software enables connection to all common industrial interfaces. FOBA Laser Marking + Engraving
FOBA Laser Marking + Engraving
www.fobalaser.comPictures for editorial use can be downloaded at: https://www.fobalaser.com/news-press/article/foba-supports-manufacturers... flexible-marking-solutions-during-the-crisis/
ALLTEC GmbH
An der Trave 27-31
23923 Selmsdorf
Germany
T +49 38823 55-0
info@fobalaser.com
www.fobalaser.comKontakte/Contacts
Dana Francksen
Director Marketing Communications
T +49 38823 55-240
dfrancksen@alltec-laser.comSusanne Glinz
Marketing Communications
T +49 38823 55-547
sglinz@alltec-laser.comImage
-
Extreme makeover of refrigerated warehou...
Cold storage retrofit challenges Americold is a third-party logistics (3PL) company serving producers, retailers and fo -
Expanded capacity for vial & syringe pa...
Amid increased demand from biologics and vaccines sectors, Company invests in additional die cutting and gluing machinery, expanding production capacity and allowing for expedited turnaround. Newark, NJ – Keystone Folding Box Co., a designer and manufacturer of paperboard packaging solutions, has incorporated new die cutting and gluing equipment into its production facility. The infrastructure investment increases the company’s production capacity of vial and syringe packaging by 25%, and accommodates shorter lead-times. Keystone offers a comprehensive line of secondary packaging systems for injectable pharmaceutical products. Available in a variety of tailored features and in both all-paperboard and plastic-hybrid formats, solutions are available for prefilled syringes, autoinjector pens, vials and ampoules, as well as combinations of these drug delivery formats. In particular, developments in the biologics and vaccines markets have led to heightened demand for custom packaging solutions for vial and prefilled syringe products. During the past six months, Keystone has seen significantly increased requests in these sectors, producing packaging solutions for pharma customers manufacturing monoclonal antibodies, vaccines, recombinant hormones/proteins, and cellular- and gene-based biologics, among other niches. According to a survey from Zion Market Research, the global vaccine market is expected to exceed $59 billion in 2020 – a figure that has nearly doubled in just six years. In the United States alone, the vaccines market is anticipated to reach $18 billion. The biologics market is growing at an even more exponential rate; according to current market reports, the global biologics market was valued at over $250 million in 2018, and is expected to exceed $625 million by 2026. To meet the building demand for packaging solutions in these sectors, Keystone’s secondary packaging collection meets a range of needs through options such as tamper evidence and child-resistance via reclosable locking mechanisms – all while ensuring parenteral product protection throughout the pharmaceutical supply chain. Solutions include: A line of Injectable Packs is suitable for prefilled syringes or autoinjector pens, and can be designed to include ancillary items like sterile wipes The InjectaSlide package incorporates a thermoformed tray that slides inside a carton and into a locked position; a lock release button must be pressed to unlock and slide the tray forward for easy access to each medicine dose. The Vial Pack line of cartons includes designs for packaging a single vial or ampoule, or as many pharmaceutical products as a program requires. “With injectable products made of glass or rigid plastic, protection is paramount.” said Ward Smith, Director of Marketing & Business Development at Keystone Folding Box Co.. “Secondary packaging must be designed and produced with the right materials to protect prefilled syringes, autoinjectors, vials and ampoules as they travel through the supply chain.” ### About Keystone Folding Box Co. While Keystone continues to be a leader in the manufacturing and design of paperboard packaging, they are also a design center and source for non-paperboard packaging components. To learn more about Keystone Folding Box Company, please contact Ward Smith at Keystone Folding Box Company, at (513) 871-4747, ward.smith@keyboxco.com or visit www.keyboxco.com. client: Keystone Folding Box Co. contact: Turchette Agency Christopher Dale (973) 227-8080, ext. 116 cdale@turchette.com Ward Smith Keystone Folding Box (513) 871-4747 ward.smith@keyboxco.comImage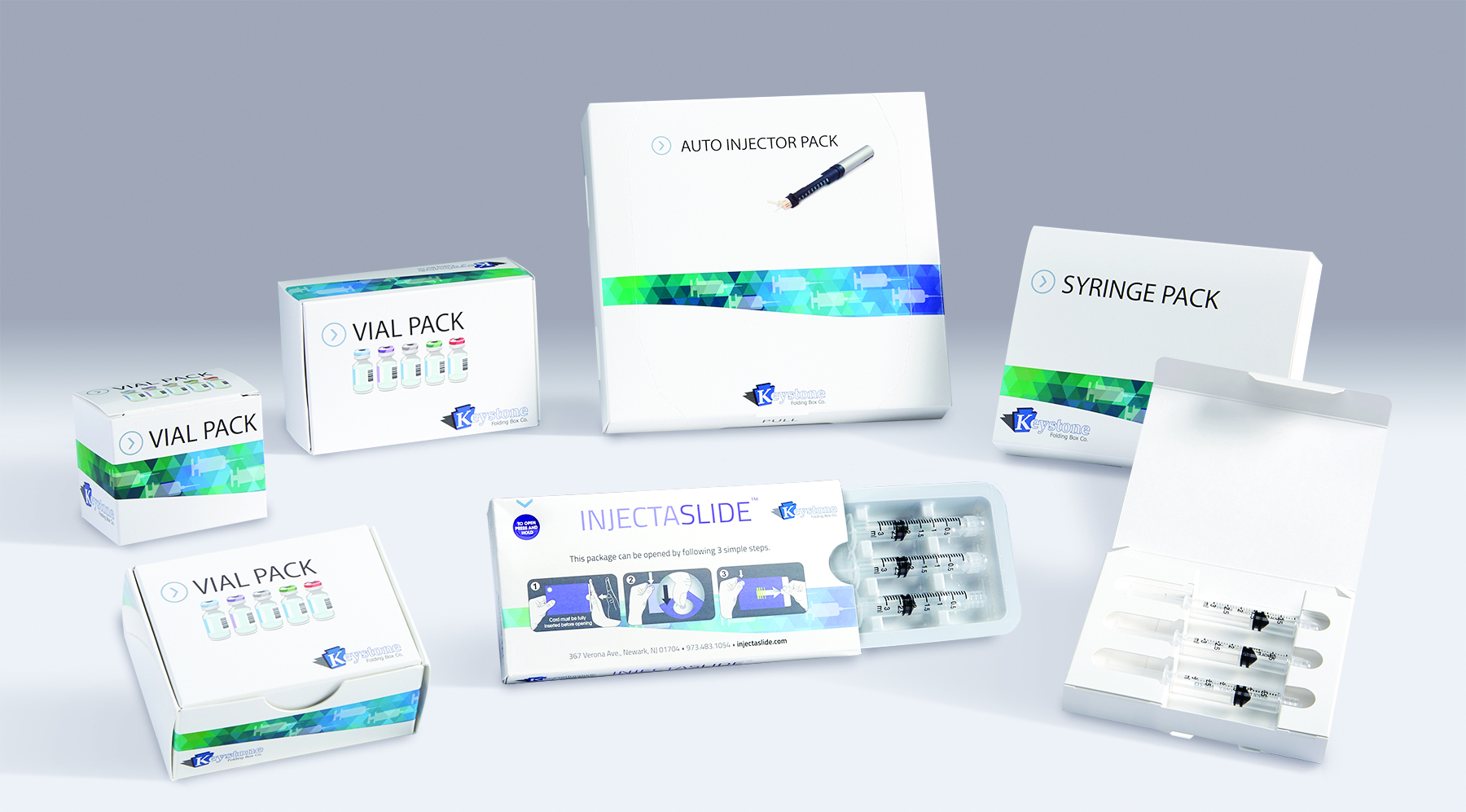
-
Legacy pharmaceutical packaging acquires...
Company also adds bottling and pouching equipment in anticipation of heightened demand. St. Louis, MO – Legacy Pharmaceutical Packaging, a contract packager serving the global pharmaceutical industry with bottling, blistering, pouching, unit-of-use, compliance and secondary packaging services, has purchased three high speed blistering and cartoning lines with serialization and aggregation capabilities from McKesson RxPak’s production facility in Memphis. The infrastructure acquisition nearly doubles Legacy’s total blister production capacity, which now stands at half a billion packs per year. In a separate equipment investment, this summer Legacy also will add an additional new high speed bottling line, bringing the contract packager’s bottling capacity to some 600 million units per year. Additionally, the company recently incorporated two new pouching machines to help meet growing demand for tablet and capsule filling applications. The expansion-minded moves come amid recent growth in both the Rx and OTC sectors, and in expectation of continued heightened demand per the company’s transition into a high-volume, one-stop shop providing turnkey product packaging straight through market delivery. Located in Memphis, McKesson RxPak is a division of McKesson Corp. and a leading provider of packaging products and services to manufacturers and retailers in the pharmaceutical industry. Michael Plumlee, Vice President of Sales “This will allow McKesson to focus on their portfolio expansion through their SKY Unit Dose Division.” “This infrastructure investment is a natural evolution of the expansion plans Legacy adopted several years ago, starting with a 100,000-square-foot expansion in 2017 that added both production capabilities and warehousing capacity providing a total of 300,000 square feet,” said David Spence, CEO of Legacy Pharmaceutical Packaging. “Considering both recent growth, and that we anticipate more US base production moving forward, this was the right time to continue in a way that makes Legacy an even more versatile, dynamic and turnkey packaging partner.” # # # About Legacy Pharmaceutical Packaging, LCC Legacy Pharmaceutical Packaging is a privately held contract pharmaceutical packaging company located in St. Louis, Missouri. Legacy is a full service pharma solution & medical device packaging provider servicing the branded, generic, government, wholesale and major retail markets. Legacy is registered with the FDA, VAWD accredited and licensed by the DEA to handle Schedule II-V drug products. Legacy Pharmaceutical Packaging is headquartered in St. Louis, MO. For more information, call (314) 813-1555 visit www.LegacyPackaging.com. client: Legacy Pharmaceutical Packaging, LLC contact: Christopher Dale Turchette Agency (973) 227-8080 ext. 116 cdale@turchette.com Brad Rayner Legacy Pharmaceutical Packaging, LLC Direct (314)549-8052 BRayner@legacypackaging.comCompany NameLegacy Pharmaceutical Packaging, LLCImage
-
Michelman joins the 4evergreen alliance ...
The three-year-long project brings together a diverse network of organizations and stakeholders throughout the value cha -
Jowat to sharpen focus on sustainability...
The new Jowatherm® GROW product range of Jowat boasts hot melt adhesives with a very high content of renewable raw mater






