A fine tradition with invercote duo
Today, Domaine Chandon is part of the LVMH group of luxury consumer brands, which includes high-end perfume and fashi
Today, Domaine Chandon is part of the LVMH group of luxury consumer brands, which includes high-end perfume and fashi
Founded in 1902 by Giulio Ferrari, these luxury sparkling wines have gained a worldwide reputation of Italian decaden
XO stands for "extra old" which is a tribute to the three-hundred-year union between Grande and Petite Champagne, loc
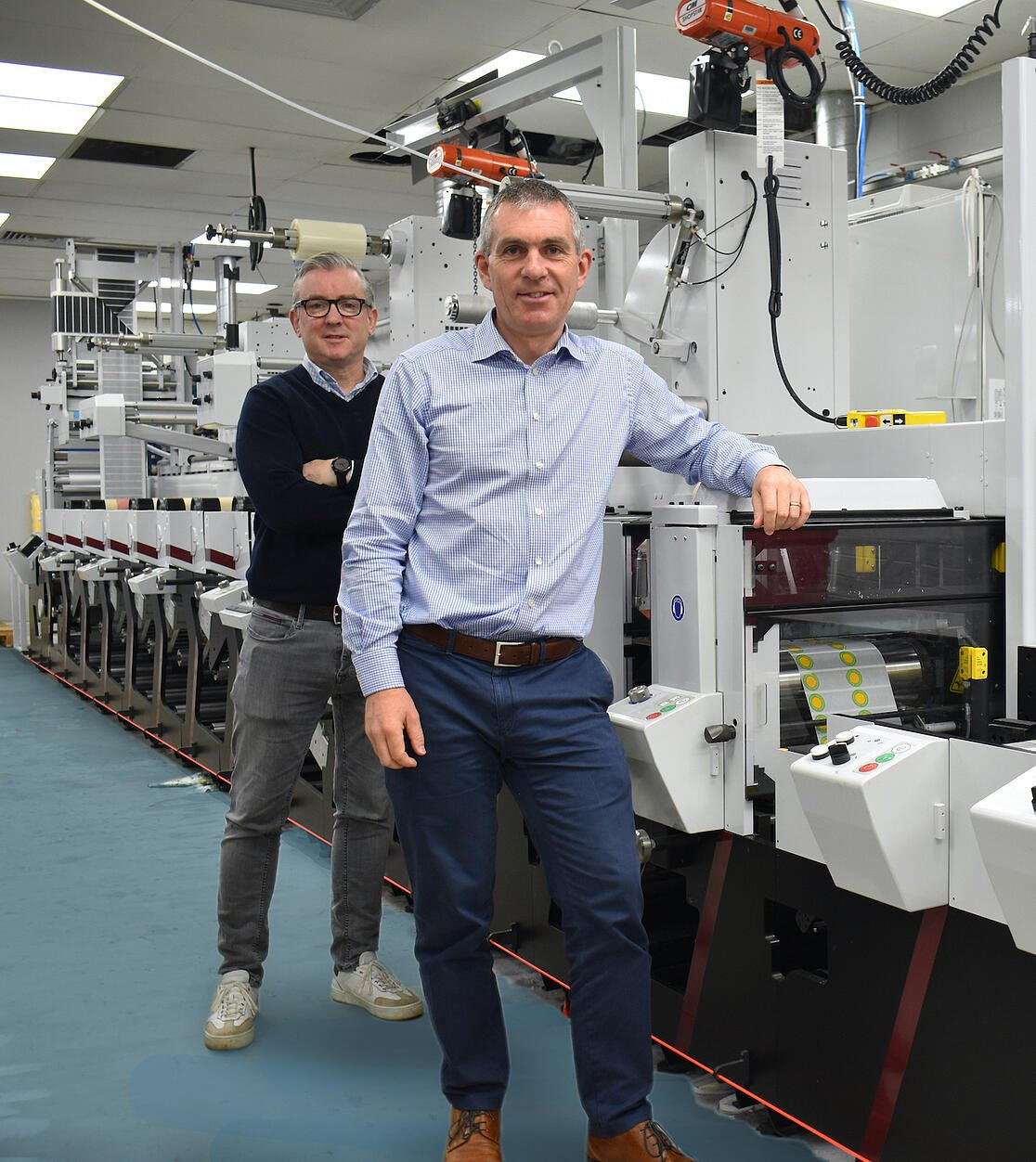
Much has changed in the self-adhesive label market since Label Tech was established in Dublin back in 1992. Today, the company is the largest independent label converter in Ireland, with a staff of 48 operating from a 20,000 sq ft facility in Santry, a northern suburb of the capital city. With multiple label print awards testifying to its prowess , Label Tech uses its knowledge and expertise to bring top quality label solutions to industry sectors including food and drink, health and nutrition, pharmaceutical, transport, and logistics.
Today the company is run by Managing Director James Costello, Sales & Finance Director Johnny Woods and Chairman David Keogh. Costello explained: “When we first opened our business, self-adhesive labels were largely functional and the demands of consumers, plus production capabilities were a long way from where they are today. In recent times, customer expectations and levels of sophistication have grown exponentially.”
As the company name suggests, Label Tech has always taken a strong interest in the technical side of printing, and significant investment over the years has ensured that it exceeds customer demands. The company has become well known for its staff’s knowledge and expertise, collaborating with customers to achieve some outstanding bespoke label solutions. Believing that a company is only as good as its people, Label Tech invests time and money in fostering the talent of its employees and encouraging them to grow their skills and competence.
“It’s the combination of this and sustained investment in key technology over the past few years that has guaranteed Label Tech has stayed ahead of the competition,” he added. The latest of these machine investments is one of the new Mark Andy Evolution flexo presses that was launched so successfully at Labelexpo in Brussels in 2019.
The Evolution, which was installed and commissioned at Label Tech over the Summer is a 13” (330mm) 8-colour press equipped with full UV curing. It has been specified with an unwind roll lift, two die stations and a sheeter and conveyor. In addition, turn bars, a cold foil facility and laminator are mounted on overhead rails, and there is a BST inspection camera. For roll-to-roll operation, a duel framed rewind with roll lift is also installed.
Chosen for its extensive production capability, the new Evolution: “is the first of three presses that will be installed over the next investment phase in Label Tech’s growth,” according to Costello, who added that the big attractions of the Mark Andy Evolution are its speed and flexibility that will serve an increasing order book and growing customer base. “Meeting and exceeding market demands is even more possible now through this machine’s versatility and scalability,” he said.
Speaking for Mark Andy, UK & Ireland Sales Manager Paul Macdonald added: “It’s another example of Label Tech putting quality and investment in the latest technology at the forefront of its business. Its increased arsenal of capability will enable customers to secure faster turnaround times and unrivalled quality on every order. Mark Andy is delighted to be playing a key part in this company’s success.”
This is the second of three Evolution presses to be installed in Ireland since the Covid-19 lockdown began in March, and Paul Macdonald is proud of the Mark Andy technicians who have worked closely with customers to ensure all health and safety issues specific to the pandemic have been strictly observed at all times.
Likewise, at Label Tech, where Level 5 Covid restrictions have been in place since 21st October, James Costello said: “Everyone here is operating with safety in mind and taking all precautions necessary to ensure that we continue to trade safely. The health and safety of our employees is of the utmost importance and we follow all guidelines and protocols provided by the HSE and the Irish Government. We are also in constant contact with our customers and suppliers to ensure compliance across all mandatory practices.”
In a year that has proved unprecedently difficult for commerce to continue, those companies with an enterprising mindset have found ways to work around the problems and build new strength into their businesses. With no immediate end in sight to the pandemic, those who adapt and continue to invest will survive and flourish at the expense of others. Label Tech exemplifies this mantra.
About Mark Andy Inc.
Mark Andy is a pioneer of the graphic arts and printing industry. As the world’s leading manufacturer of narrow- and mid-web printing and finishing equipment, it supplies leading global brands, including Mark Andy and Presstek printing presses, Rotoflex finishing solutions, as well as a complete line of Mark Andy Print Products consumables and pressroom supplies. All products are backed by the largest customer support team in the industry, minimizing downtime and helping customers be profitable, efficient and at the forefront of innovation. Mark Andy does what it takes to understand each customer’s unique business, the challenges they face and the pressures they feel. It strives to provide products and services that help customers solve their problems and solutions that go a step further, allowing them to excel in their day-to-day operations, ultimately increasing productivity and reaching their full potential. For more information, visit www.markandy.com.
For additional media information,
please contact
Amanda Flower
+1 636 681 9036.
We are working in strict compliance with all prescribed health regulations in order to protect our employees’, suppli
The line is capable of producing up to 120 (60+60) pillow bag packs per minute from 100 to 500 gr and more.
Cambridge, November 18, 2020: The results of a series of lab experiments demonstrate why Xaar’s TF Technology ink recirculation, invented more than a decade ago, still sets the benchmark for industrial inkjet recirculation techniques today to deliver a wider window of operation for users.
Presented in Xaar’s new white paper subtitled ‘What goes around, comes around’, the research compares Xaar’s TF Technology ink recirculation with alternative ink recirculation techniques used by most other printhead manufacturers.
Whilst ink recirculation is now commonplace, the white paper clearly shows that all technologies are not equal. A printhead’s architecture determines how well ink recirculation is implemented and therefore influences the degree to which the method delivers benefits across today’s wide range of printing and jetting applications.
TF Technology is a key building block in Xaar’s recently announced ImagineX bulk platform which will power the next 30 years of innovation in inkjet capabilities and printhead launches.
Together with the unique Hybrid Side Shooter printhead architecture, Xaar’s TF Technology enables ink or other fluids to flow directly past the back of the nozzle during drop ejection at very high flow rates.
This ensures the nozzles are continuously primed, keeping the printhead operational and the nozzles firing and – with the ink in constant motion – prevents sedimentation and nozzle blocking, particularly in heavily pigmented inks. Any air bubbles and unwanted particles in the ink are also carried away, improving reliability, even in the harshest industrial environment.
With TF Technology, jetting is significantly more reliable compared to alternative ‘roof mode architecture’ design printheads whose convoluted ink flow paths means that recirculation is close to, but not at the back of the nozzle.
“Xaar’s TF Technology ink recirculation completely revolutionised inkjet printing and has become an essential feature for a wide range of industrial inkjet applications,” said Angus Condie, Director of Technology at Xaar and one of the authors of the white paper.
Launched by Xaar in 2007 with the introduction of the Xaar 1001 printhead XF Technology has since opened up exciting new applications such as glass printing with frit inks, high opacity white pigment ink printing for labels, and heavily loaded conductive inks for printed electronics - transforming the digital inkjet industry and becoming an industry requirement for effective and reliable inkjet printing.
“Our research proves that ink recirculation solutions vary due to their architectural design, yielding vastly different results. Xaar’s TF Technology still greatly outperforms its rivals and continues to set the benchmark for ink recirculation today, delivering the widest window of operation within inkjet printing,” concluded Condie.
The complete white paper can be downloaded from Xaar’s website and is available now at https://www.xaar.com/en/resource-centre/tf-technology-what-goes-around-….
Ends
Image shows a Xaar 2002 printhead with TF Technology in manufacture.
About Xaar
Together with our partners and customers, Xaar has been transforming the world of inkjet technology for 30 years – and we’re just getting started.
With a new leadership team, new technology and new products, we have an exciting roadmap for the future – that will help our partners unleash the true power of inkjet printing and open up a world of opportunities for their business. We believe true innovation comes from collaboration, which is why all our teams work together in Cambridgeshire where industrial inkjet was born.
Collaboration is at the core of our global partnerships too - whether we’re helping customers enhance their uptime or create production efficiencies from high-speed digitisation - we’re always innovating together.
We know that inkjet technology can not only maximise the efficiency of our partners’ businesses but help them evolve too. That’s why we go on a journey with our customers - offering expert insights and technical support every step of the way. Just like our printheads, Xaar’s partnerships are built to last.
Welcome to a whole new Xaar. www.xaar.com
Contacts:
Xaar: Charlotte Baile
T: +44 1223 802151
E: charlotte.baile@xaar.com
Global ex China/USA: Nielsen McAllister, Simon Wildash / Tom Erskine
T: +44 1332 293939
E: info@nmpr.co.uk
China: CommNow, Qianzi Che,
T: +86 10 5096 1513
E: qianzi.che@commnow.cn
USA: Press+, Irvin Press
T: +1 508-384-0608

Aptar Beauty + Home, a global leader in dispensing and full packaging solutions for the beauty, personal care and home care industry, has taken a further step toward its sustainability goals by pre-qualifying 45+ products to ensure alignment with Credo’s Sustainable Packaging Guidelines. The current Credocompliant product selection includes airless full packaging, dispensers, spray technology and the broadest range of dispensing closures available in at least 50%, but up to 100%, post-consumer recycled resin*, in the North America market.
Credo, a pioneer in the clean beauty category, released its Sustainable Packaging Guidelines in April 2020 in response to the growing concern about beauty packaging waste. These guidelines are the latest chapter of The Credo Clean Standard™ which defines “Clean Beauty” and offers brands a comprehensive playbook on safety, sourcing, sustainability and more. According to Google Trends, consumer interest in sustainable brands has almost doubled in the past year** as more consumers were able to see the first-hand environmental impacts through pandemic shut downs.
“As a leader in the packaging industry our mission is to help our brand partners on their sustainability journey while working towards our own circular economy goals. By pre-qualifying our sustainable products, produced in North America, in alignment with Credo’s guidelines, we hope to facilitate easier sourcing and provide clear guidance on our products’ environmental impacts.” - Philippe Erhart, President of Beauty + Home, North America
With the introduction of the Sustainable Packaging Guidelines, Credo will become the first beauty retailer to introduce a mandatory packaging guideline for all of its brand partners to follow. The policy will:
Prohibit single-use masks and wipes by June 2021
Require brands to replace virgin petrochemical plastic with 50% or more recycled plastic, or another non-plastic material, by June 2023
Disallow brands from implying packaging is compostable or recyclable if it is not; brands must provide clear, accurate disposal instructions for consumers in an effort to help improve the
overtaxed, contaminated recycling stream
Emphasize reusable packaging systems: durable containers the customer keeps and refills with new product, in packaging designed to be environmentally preferable
“We are at a crucial point with regard to consumption and waste. It is time that beauty companies make real, actionable commitments that will drastically reduce this sector’s impact,” said Mia Davis, Credo’s Director of Environmental and Social Responsibility. “Credo’s Sustainable Packaging Guidelines offer brands clear goals and deadlines for better material choices, and as a part of this work, we’re forging new, pre-competitive alliances with packaging companies like Aptar to ensure access to better, more sustainable materials.”
Aptar’s global approach to sustainability addresses multiple facets from a people, product and planet perspective. In alignment with the Ellen MacArthur Foundation’s New Plastics Economy Global
Commitment, Aptar has committed to 100% of Beauty + Home’s products will be recyclable, reusable, or compostable by 2025. Recently, Aptar announced formalizing and approval of its Science Based Targets, setting an emissions goal consistent with requirements to keep global warming below 2° Celsius by 2030. This implementation incorporates operations electricity, fuel oil, natural gas and refrigerant use as well as operations including transportation of goods and raw materials. Click here for additional information.
###
About Aptar Beauty + Home:
Aptar Beauty + Home is part of AptarGroup, Inc., a global leader in the design and manufacturing of a broad range of drug delivery, consumer product dispensing and active packaging solutions. Aptar uses insights, design, engineering and science to create dosing, dispensing and protective packaging technologies for the world’s leading brands, in turn making a meaningful difference in the lives, looks, health and homes of millions of people around the world. Aptar’s innovative solutions and services serve a variety of end markets including pharmaceutical, beauty, personal care, home, food and beverage. The company is headquartered in Crystal Lake, Illinois and has 14,000 dedicated employees in 20 countries.
For more information, visit www.aptar.com.
About Credo Beauty
Founded in 2014 by former Sephora executives, Credo is today’s largest and fastest-growing clean beauty retailer, partnering with 135+ beauty brands across skincare, color, body, fragrance and hair care categories. Credo’s mission continues to be focused on changing the beauty industry for the better and making clean beauty accessible to everyone. All brands must comply with the Credo Clean Standard, the most comprehensive in beauty retail. With an omni-channel approach, Credo currently boasts nine stores across the U.S. and an ecommerce site experiencing extraordinary growth. With transparency and education at the forefront, customers and brands alike benefit from Credo’s services/programs such as Credo Live (online and video chats with in-store reps), Clean Swaps Program, Your Product Finder (smart database to navigate 450k ingredients), local retail partnerships/education panels/event series, and access to our Clean Beauty Council (industry experts leading the Clean Beauty movement). A portion of every sale goes to the non-profit partner, Lipstick Angels.
Media Contacts:
Jaimee Given
Senior Marketing Communications Manager
Aptar Beauty + Home, North America
Jaimee.Given@aptar.com
Jessica Fitzsimons
Senior PR Manager, Credo Beauty
Jessica@CredoBeauty.com

New dimensions and products available from stock
‘Tackling antimicrobial resistance is a global challenge and a high priority for EMA and the European medicines regul
Mondi scoops six competency awards across three categories at the Turkish Crescents and Stars for Packaging 2020 Competition
Products from both corrugated and flexible packaging teams recognised
16 November 2020 – Mondi, a global leader in packaging and paper, has scooped six competency awards in the prestigious Turkish Crescents and Stars for Packaging 2020 Competition, organised by the Turkish Packaging Manufacturer’s Association.
The company’s innovative approaches to sustainable and effective packaging solutions were recognised in four different categories:
Industrial and Transportation
Cable Reel from Mondi Adana Plant
FloralBox from Mondi Tire Plant
Flexible Packaging
Unilever Recyclable PP Base Soup Packaging from Mondi Kalenobel
Orkla Metal Free Structure for Chips from Mondi Kalenobel
Food
Safe Bottle Box from Mondi Karaman Plant
Point of Sale, Presentation and Storage Products
One Piece from Mondi Izmit Plant
Almost 280 different packaging products were assessed across the 13 award categories in the ninth Crescents and Stars for Packaging competition. The award wins demonstrate Mondi’s cross-market approach to packaging, spanning a range of industries and serving very diverse business and consumer audiences.
Sevinç Yener Çimecioğlu, CEO Mondi Tire Kutsan said: “The standard of entries was very high, so we are delighted to receive these awards. The Crescents and Stars for Packaging competition credits the way we combine creativity and technology to help our customers achieve their sustainability goals, finding ideal, unique packaging solutions for their goods. We are contributing to a better world by making innovative solutions using paper where possible, plastic when useful.”
Umit Sahin, Managing Director, Mondi Kalenobel added: “It is fantastic to be recognised for the sustainable packaging we created in collaboration with our customers Orkla and Unilever. This is testament to the hard work of our teams and the strong relationships we enjoy with our global clients. Our aim is always to work closely with our valued partners through our customer-centric approach, EcoSolutions, to create sustainable packaging that works for the products, consumers and the environment.”
The winners of the Crescents and Stars for Packaging Competition 2020 will be presented with Gold, Silver and Bronze trophies at a later date, once large scale gatherings are permitted due to the pandemic.
/ends
Cable Reel: Cable Reel is a functional two-pieces reel design made of corrugated cardboard. It is used for wrapping of plastic cables or wire. This environmentally-friendly solution is a sustainable substitute for plastic wrapping.
FloralBox solution: FloralBox is an e-commerce solution offering a modular system to securely ship potted plants of different types and sizes and in various combinations. The one-for-all insert accommodates different protection needs, with consumers delighted by the pristine condition the plants arrive in alongside the positive unboxing experience.
https://www.mondigroup.com/en/products-and-solutions/trends/e-commerce/…;
Unilever Recyclable PP Base Soup packaging: A recyclable mono-material film product was created for Knorr’s dry soup powder range, replacing a multi-material laminate. The packaging preserves the shelf-life of the food product and works with existing production machines. The product was developed by Mondi to help Unilever stay committed to using plastic packaging that is 100 per cent reusable, recyclable and compostable by 2025.
https://www.mondigroup.com/en/newsroom/press-release/2020/mondi-support…;
Orkla Metal Free Structure for Chips: Mondi’s metal-free high barrier laminate makes the packaging for Orkla Confectionery and Snacks Norway`s chips range recyclable by eradicating the need to include a metalised layer, retaining crispness and avoiding grease leakage.
Safe Bottle Box: Safe Bottle Box is designed to protect a single bottle from any impact during the e-commerce shipments. Thanks to the adjustable flap on the inner box, it can be adapted to bottles of different sizes.
One Piece: One Piece is an exhibition/display and transportation unit that provides advantages to the end consumer as well as to the manufacturer with its simple, shopping-friendly structure and its one-piece design.
About Mondi
Mondi is a global leader in packaging and paper, contributing to a better world by making innovative, packaging and paper solutions that are sustainable by design. Our business is fully integrated across the value chain – from managing forests and producing pulp, paper and plastic films, to developing and manufacturing effective industrial and consumer packaging solutions. Sustainability is at the centre of our strategy and intrinsic in the way we do business. We lead the industry with our customer- centric approach, EcoSolutions, where we ask the right questions to find the most sustainable solution. In 2019, Mondi had revenues of €7.27 billion and underlying EBITDA of €1.66 billion.
Mondi has a premium listing on the London Stock Exchange (MNDI), and a secondary listing on the JSE Limited (MNP). Mondi is a FTSE 100 constituent, and has been included in the FTSE4Good Index Series since 2008 and the FTSE/JSE Responsible Investment Index Series since 2007.
Contact:
Judith Wronn
Senior Communication Manager, Flexible Packaging & Engineered Materials
Tel: +49 151 1771 4692
Email: Judith.Wronn@mondigroup.com
Josina van der Velden
EMG
Tel: +31 164 317 014
Email: jvandervelden@emg-marcom.com

Mitsubishi HiTec Paper and 13 other companies from the chemical and paper industry in North Rhine-Westphalia save a further 113 million kilowatt hours of energy and thus over 36,000 t of CO2 per year.
The Energy Efficiency Network ChePap Rhein-Ruhr has again drawn a positive balance. After considerable energy savings were achieved through close cooperation between 2016 and 2018, the second period from 2018 to 2020 also produced positive results with the 14 companies from the NRW chemical and paper industry. In addition to the 158 million kilowatt hours of energy saved per year from the first term, additional 113 million kilowatt hours per year have now been saved. The savings from the current round correspond to a CO2 reduction of 36,477 tons per year – which is after all the CO2 footprint of 3,500 German citizens.
This great success shows that it is worthwhile to approach and implement innovations as part of a network. Because one thing is clear: Many obvious savings have been implemented in energy-intensive industries such as the chemical and paper industries for years. However, further synergies can be achieved through mutual exchange between companies and industries. The energy efficiency network initiative of the federal government with business associations offers an excellent basis for this.
Hans-Jürgen Mittelstaedt, Managing Director VCI NRW says: “The large savings again clearly show how valuable the network continues to be for the companies represented. We are therefore planning to extend ChePap for a further two years. In the near future in particular, many companies will have to start working towards greenhouse gas neutrality. It is important to be able to discuss ideas and possible solutions with colleagues in the network. I am looking forward to ChePap III. "
Martin Drews, Managing Director of the Paper Association of North Rhine-Westphalia, adds: “The exchange beyond industry boundaries offers a decisive added value for the participants. Companies from the paper industry can benefit from innovative savings opportunities and business ideas from the chemical industry and vice versa. We therefore welcome the continuation of the successful cooperation in the coming year. "
Gerd Finkenhofer, Energy Manager at Mitsubishi HI Tec Paper, explains: “In addition to the specific energy-saving goals and results that are required for the national energy transition to succeed, I particularly value the cross-sector networking. This year, all energy-intensive companies had to convert their energy management systems to a revised standard in accordance with DIN EN ISO 50001: 2018. Targeted specialist presentations at our network meetings and the forum for exchange were therefore of great importance to me. At the beginning of October, we were able to successfully implement the desired recertification of the energy management system, which was first certified in 2011."
Energy efficiency networks such as ChePap Rhein-Ruhr are associations of companies that develop and implement company-specific measures to save energy in a joint exchange. The specialists of the member companies are supported by experienced energy consultants, in the case of ChePap Rhein-Ruhr II from the Aachen engineering firm WiRo. The network is supported by the trade association of the Rhenish-Westphalian Paper-Producing Industry and the North Rhine-Westphalia Chemical Industry Association. The following companies are part of the ChePap Rhein-Ruhr II Energy Efficiency Network: Dralon GmbH, Grillo-Werke AG, INOVYN Deutschland GmbH, Kabel Premium Pulp & Paper GmbH, Martinswerk, Mitsubishi HiTec Paper Europe GmbH, OQ Services GmbH (formerly Oxea), paper mill Niederauer Mühle GmbH, Reflex GmbH & Co. KG, SABIC Polyolefine GmbH, Topas Advanced Polymers GmbH, Versalis Germany, Vestolit GmbH, WEPA Industrieholding SE.
Mitsubishi HiTec Paper Europe GmbH is a German subsidiary of Mitsubishi Paper Mills Ltd., Japan, one of the world's leading manufacturers of specialty paper. The roughly 780 employees at Mitsubishi HiTec Paper Europe produce high-quality direct thermal, inkjet, carbonless, label and barrier papers at two tradition-rich locations in Bielefeld and Flensburg. Each factory stands out for own base paper production, state-of-the-art production machinery and innovative coating technologies. Through its dense global sales network, Mitsubishi HiTec Paper Europe supplies a full range of specialty papers for many applications and printing technologies – and is a highly capable partner whenever customized coated paper solutions are required.
Contact:
Ralf Buhl
Marketing Manager
Mitsubishi HiTec Paper Europe GmbH
Niedernholz 23 | 33699 Bielefeld | Germany
Tel. +49 (0)521 2091-582
ralf.buhl@mitsubishi-paper.com
www.mitsubishi-paper.com
TÜV Rheinland certifies that the vacuum pump is completely free of oil of the best "Class 0"
Cologne, November 2020: With the new oil-free DSS scroll vacuum pump, Atlas Copco is expanding its range of dry industrial pumps. The robust, low-wear pump is particularly suitable for vacuum generation in the rough vacuum range. A key feature of the innovation is its simple and effective operating principle for gas handling. Inside the pump there are two intermeshing, spiral-shaped screws made of aluminium. One spiral screw is fixed, while the second one rotates to compress the gas inclusions.
Lower life cycle costs
The ergonomic vacuum pump is also characterized by low energy consumption, lower life cycle costs and user-friendly operation. Due to the dry running of the pump, no oil changes are necessary; there is also no need to replace the exhaust filters. Therefore the
overall maintenance requirements are relatively low. The reduced service and maintenance costs can also be attributed to the fact that the removable front cover simplifies access to the pump interior of the DSS scroll vacuum pump.
Predestined for pharmaceutical and food applications
Equipped with these properties and high-quality materials, the new development is particularly suitable for applications in the pharmaceutical industry as well as in food packaging and processing. In these and other industries, the pump will provide a stable vacuum at an atmospheric pressure of up to 1 mbar and maximum productivity at low to medium flow rates.
According to TÜV Rheinland "completely oil-free
"Another important feature of the DSS scroll vacuum pump is its complete freedom from oil. It does not use any oil for sealing or cooling, so there is no risk of oil contamination," explains Alexander Frerichs, the responsible Atlas Copco Product Manager. To verify and prove this, TÜV Rheinland has carried out extensive measurements of the aerosol oil content in 2019. "No traces of oil whatsoever could be detected in the exhaust air stream, which is a maximum of cleanliness and safety for the production environment and people," emphasizes Alexander Frerichs. Based on the TÜV results, Atlas Copco's new DSS scroll vacuum pump has been classified and certified in the top "Class 0".
Exemplary applications at a glance:
- Processing, packaging, drying of food
- Thermoforming
- Medical Systems
- Vacuum impregnation
- Central house vacuum
- Pick and place
- Laboratory vacuum.
Atlas Copco Vacuum Technique
Great ideas accelerate innovation. At Atlas Copco Vacuum Technique we collaborate with our customers to turn industrial ideas into leading edge technology in vacuum and abatement solutions. Our passionate people, expertise and service bring sustainable value to industries everywhere. Atlas Copco is based in Stockholm, Sweden with customers in more than 180 countries and about 37 000 employees. Revenues of BSEK 95/ 9 BEUR in 2018.
At Atlas Copco Industrial Vacuum, we have revolutionized vacuum technology. Our state-of-the-art vacuum pumps and systems exemplify today’s connected and digitalized industry. Our teams of exceptional and passionate people engineer customer-centric vacuum solutions that offer better energy efficiency, consumer safety, improved productivity and a sustainable future. Our products are the invisible force that drive all industrial applications and manufacturing.
Part of the Atlas Copco Vacuum Technique business area, we are headquartered in Cologne, Germany with production centers in Germany, France, Belgium, Czech Republic, USA and China.
For more information please contact:
Alexander Frerichs, Product Manager - Dry Pumps Atlas Copco Vacuum
+31(0)6-15349311, alexander.frerichs@atlascopco.com
Christoph Angenendt, Communications Manager Industrial Vacuum Division
+49 (0)172 29 650 75, Christoph.Angenendt@vt.atlascopco.com

Henkel and Greiner Packaging have received a special distinction, with their innovative and sustainable packaging solution for Persil 4in1 Discs coming out a winner at the World Packaging Organisation’s Green Packaging Award 2020.
Kremsmünster, Austria, November 2020. The sustainable cardboard-plastic packaging used by Henkel for its Persil 4in1 Discs has been continually upgraded in recent years and is now made from 50 percent postconsumer r-PP. A jointly developed solution, the packaging was a winner at the Green Packaging Award 2020.
“Greiner Packaging has been producing sustainable solutions for many years, and this was a key factor for the award in the Eco-Friendly Company Philosophy category. The new Persil 4in1 Discs packaging solutions are an example of the ongoing collaboration between our companies in this area. So we are delighted to receive this award together with our longstanding customer Henkel,” says Axel Kühner, CEO of Greiner AG.
Sustainable packaging solution
The partners’ ongoing joint development efforts focused on improving the sustainability characteristics of an existing cardboard-plastic solution for premeasured detergent products by using 50 percent postconsumer r-PP. From this perspective, the new K3®-F cardboard-plastic combination is especially impressive because it links high-quality packaging with appealing marketing communication opportunities and a positive environmental impact. This smart combination boasts a lower weight and helps save up to 40 percent more plastic than the previous packaging. Incorporating 50 percent recycled plastic halves the amount of virgin material used. As a result, the 4in1 Discs packaging stands out not just for its sustainability profile but also in terms of its technical functionality.
“We have set ourselves ambitious goals for sustainable packaging and want to promote a circular economy throughout the value chain together with our partners. The use of recycled material plays a key role in this – and the innovative packaging for the Persil 4in1 Discs is a good example of the progress we have already made toward this goal: We use over 90 percent recycled cardboard for the sleeve, and the plastic containers are made from 50 percent r-PP, a recycled material. Production of the new packaging involves a two-stage process. We make the inside of the plastic container from white virgin material, which guarantees a high degree of color contrast with the colorful detergent discs. The packaging’s outer coating, meanwhile, contains recycled plastic obtained from end consumer households. This layer’s gray color does not impact the packaging’s look, because it is wrapped in an attractively printed cardboard sleeve,” explains Eduardo Celada, international packaging manager for laundry care at Henkel.
The sustainable containers for the premeasured detergent products are also more effective in terms of recycling. Thanks to the innovative, patented tear-off system, the cardboard sleeve and plastic container can be easily separated and disposed of on their own by consumers. This makes them 100 percent recyclable. The packaging’s lower plastic content also helps to reduce CO2 emissions, while the cardboard wrap maintains the container’s stability.
Perfect design solution for global brand owners
The plastic containers and folded cardboard sleeves for the K3®-F packaging are delivered separately to Henkel. The sleeves are only folded by a machine and slipped over the container directly before filling at the company. This process is an excellent fit for global brand manufacturers who offer their product in various markets or under different brands and therefore work with a wide range of decoration solutions. This gives them a high degree of flexibility in terms of the design and keeps stocks to a minimum.
Packaging facts:
- Material: PP
- Technology: Thermoforming
- Decoration: Cardboard sleeve
About Greiner Packaging
Greiner Packaging is a leading European manufacturer of plastic packaging in the food and nonfood sectors. The company has enjoyed a reputation for outstanding solutions expertise in the fields of development, design, production, and decoration for 60 years. Greiner Packaging responds to the challenges of the market with two business units: Packaging and Assistec. While the Packaging unit stands for innovative packaging solutions, the Assistec unit focuses on producing custom-made technical parts. Greiner Packaging employs a workforce of around 5,000 at more than 30 locations in 19 countries around the world. In 2019, the company generated annual sales revenues of EUR 690 million (including joint ventures), which represents more than 40 percent of Greiner’s total sales.
About Henkel
Henkel operates globally with a well-balanced and diversified portfolio. The company holds leading positions with its three business units in both industrial and consumer businesses thanks to strong brands, innovations, and technologies. Henkel Adhesive Technologies is the global leader in the adhesives market – across all industry segments worldwide. In its Laundry & Home Care and Beauty Care businesses, Henkel holds leading positions in many markets and categories around the world. Founded in 1876, Henkel looks back on more than 140 years of success. Henkel generated sales of over EUR 20 billion in the 2019 financial year and recorded an adjusted operating profit of EUR 3.2 billion. Henkel employs more than 52,000 people globally – a passionate and highly diverse team, united by a strong company culture, a common purpose to create sustainable value, and shared values. As a recognized leader in sustainability, Henkel holds top positions in many international indices and rankings. Henkel’s preferred shares are listed on the DAX stock exchange. For further information, see www.henkel.com.
Caption:
Proud winners of the Green Packaging Award 2020 for Persil 4in1 Discs packaging: Axel Kühner, CEO, Greiner AG and Birgit Rechberger-Krammer, Corporate Senior Vice President for Laundry & Home Care in Europe, Henkel.
Please direct any questions to:
Roland Kaiblinger I Account Executive
SPS MARKETING GmbH | B 2 Businessclass | Linz, Stuttgart
Jaxstrasse 2–4, 4020 Linz, Austria
+43 (0) 732 60 50 38-29
r.kaiblinger@sps-marketing.com
www.sps-marketing.com/en
Greiner Packaging International GmbH
Greinerstrasse 70, A-4550 Kremsmünster, Austria greiner-gpi.com

Schreiner Group based in Oberschleissheim (Germany) has previously received numerous national and international awards for Schreiner MediPharm’s Needle-Trap. Now the innovative needle protection system has won recognition for the first time also in the Middle Kingdom: In the competition of the Chinese packaging and printing industry association, it was selected for a Gold Award in the “Labels” category.
The China Packaging Federation (CPF) and the Pharmaceutical Packaging Printing Committee of the China National Pharmaceutical Packaging Association (CNPPA) have been organizing the competition since 2014. Divided into various categories such as food and semi-luxury food packaging, soft packaging and labels, the product entries are evaluated under the aspects of outstanding design, technical innovation, printing technology and printing quality. In the “Labels” category, Needle-Trap won a first price in the competition.
The awards ceremony took place in September as part of the “Suzhou Dialogue” event in Suzhou (Jiangsu province, west of Shanghai). This annual conference is organized by the CNPPA. Jamie Long, General Manager of Schreiner Group at the company’s Chinese location in Fengpu near Shanghai, accepted the prize: “‘Made in Germany’ is highly valued in our country because German products have a reputation of trustworthiness and reliable performance. I’m very proud that Needle-Trap, which was developed at our German headquarters more than ten years ago, has won in a Chinese competition.”
Needle-Trap is a unique, active needle protection system for prefilled syringes. It consists of a label-integrated needle trap that secures the syringe needle after an injection. This mechanism helps prevent accidental needlestick injuries. More than 1 billion Needle-Traps have been produced since the product’s market launch in 2009. It is used for prefilled syringes by renowned pharmaceutical manufacturers in Europe, North and South America, Asia and Africa.
For more information, please contact:
Susanne Höppner,
Corporate Communications
Phone +49 89 31584-5852,

Forker has held the position for more than 21 years. He will be succeeded by Dr. Nicolas Wiedmann (47), who previously held the position of General Manager for the Business Segment Special Applications and Member of the Executive Board of the Business Division Aftermarket and Special Applications at Hella GmbH & Co KGaA. He will join Siegwerk on January 1, 2021, and will take over full responsibility following the transition period and Forker’s departure at the end of March.
Wiedmann brings many years of experience in managing global businesses and implementing change processes. Apart from his most recent position at the automotive supplier Hella GmbH & Co KGaA, he held executive roles at Kautex Textron GmbH & Co. KG, a polymer processing company producing plastic packaging amongst others, and Johnson Controls Automotive Europe in Germany. The PhD physicist has successfully built up innovation and product roadmaps, led merger and acquisition activities and introduced new customer and Supply Chain Management strategies to ensure efficient and sustainable business growth. During his tenure at McKinsey & Company in Duesseldorf, he led international consultant and client teams as Engagement Manager, focusing on automotive, high-tech and TIME industries (telecommunications, information technology, media and electronics).
“I am excited about the opportunity to join Siegwerk at such an important time in its long history as we prepare the company, and our employees, our Siegwerkers, for the future,” said Wiedmann. “I plan on continuing this transformation process which Herbert Forker has led and to seize new opportunities in the dynamic packaging industry. I am also looking forward to meeting our more than 5,000 Siegwerkers around the world soon and getting to know my new leadership team.”
Forker joined Siegwerk in 1999. He has proven himself a visionary in the packaging ink industry. Through numerous acquisitions, a strong focus on innovations in packaging inks and sustainability and an early adopter of disruptive digital strategies, Siegwerk is well positioned for its strategic direction as a “global circular and digital packaging solutions provider.”
By consistently transforming Siegwerk and by rapidly adapting to changing market developments in the packaging ink segments under Forker’s leadership, the family-owned company has more than quadrupled both sales and profitability.
In 2016, Forker and Siegwerk owner Alfred Keller were named “Entrepreneurs of the Year” by Ernst & Young. “It has been my honor to serve the Siegwerk family for more than two decades,” said Forker. “I am proud of what we all have achieved as a company. Successfully implementing our current strategy to be a circular and digital packaging solutions provider is an important step in remaining globally competitive. I wish Nicolas Wiedmann and the Siegwerk team all the best and will support him any way I can to ensure a smooth leadership transition and uninterrupted business continuity.”
Picture: Dr. Nicolas Wiedmann
About Siegwerk
Siegwerk, a sixth-generation family-owned company, is one of the leading international manufacturers of printing inks and individual solutions for packaging, labels, and catalogs. With more than 180 years of experience, the company has solid expertise in and knowledge of many printing procedures. A global manufacturing and service network ensures customers consistently high-quality products and services. In keeping with the company’s philosophy “Ink, Heart & Soul,” Siegwerk seeks long-term cooperation with its business partners. Siegwerk employs some 5,000 people worldwide in more than 30 country organizations and is headquartered in Siegburg near Cologne. Further information on Siegwerk can be found at www.siegwerk.com
Media Contact:
Dr. Bettina Horenburg
Director Corporate Communications
Tel.: +49 2241 304-732
E-mail: press@siegwerk.com

The efficiency and sustainability benefits that can be achieved with the latest tray sealing solutions will be the major focus of Proseal’s participation in this year’s Asia Fruit Logistica-ON, where the company will be exhibiting as part of JBT Corporation.
In particular, Proseal will highlight how its machines are able to ensure seal quality and maintain product freshness while using the minimum amount of plastic necessary.
In the UK, Europe and North America, Proseal has been the driving force behind the replacement of traditional clam-shell punnets with top film tray sealed varieties, which reduces plastic consumption by up to 45%. With the introduction of non-plastic materials for trays, such as cardboard and the latest recyclable or home compostable materials, plastic usage can be further reduced by as much as 96%.
In addition, Proseal can offer additional plastic reductions with its unique Reflex Tooling™ which provides savings through the reduction of the skeletal film waste of ~11%.
All Proseal tray sealers feature a rugged and hygienic food industry approved construction with washdown protection. A user-friendly menu driven control panel with step-by-step prompts, error and status displays, and batch pack counter ensures ease of set-up.
Proseal equipment will be shown alongside the wide range of equipment from JBT which is geared to fresh produce, including fruit, exotic fruit, vegetables, and prepared meals.
The company’s post-harvest solutions can help to preserve the appearance and quality of produce and apply Natural Branding© using laser technology by LaserFood. Its FTNON products provide robotic and streaming technologies, helping to prepare produce for packaging or further processing including, washing, de-coring, peeling, slicing and mixing.
JBT is also the world leader in advanced fruit and juice processing solutions, with over 75% of the world’s citrus juices produced on JBT extractors. Its extensive range of equipment enables fruits, tropical fruits and vegetables to be converted to purees, concentrates, nectars, or single-strength juice. The equipment covers extraction to filling as well as aseptic processing and AVURE technologies for High Pressure Processing (HPP).
Founded in 1998, Proseal designs and manufactures high quality tray sealing machines, conveyor systems and sealing tools for food industry markets worldwide. Proseal is part of the JBT family, a leading global technology solutions provider to high-value segments of the food processing industry, committed to providing a service that surpasses customer expectations. Alongside Proseal, the JBT presence at Asia Fruit Logistica-ON also includes FPT and FTNON.
Ends
EDITORIAL ENQUIRIES:
Bob Bushby
Nielsen McAllister PR Limited
Tel: +44 (0) 1332 293939
Email: info@nmpr.co.uk
READER ENQUIRIES:
Proseal UK Ltd –
Tel: +44 (0) 1625 856 600
Email: info@prosealuk.com
Proseal America Inc –
Tel: +1 804 447 9038
Email: info@prosealamerica.com
Proseal Australia Ltd –
Tel: +61 (0) 3 9397 0955
Email: info@prosealaustralia.com
Website:
www.proseal.com
www.jbtc.com

ILPRA presentsFoodpack Hyper, the new line of heat sealing machines for packaging, designed to satisfy high productio