Nuria plans, new commercial director of ...
Nuria Plans, LIC & MBA (ESADE, Barcelona and HEC, Paris), has just been appointed the new Commercial Director of
Nuria Plans, LIC & MBA (ESADE, Barcelona and HEC, Paris), has just been appointed the new Commercial Director of
This new development is preceded by the need to improve one of its current pick and place systems, cataloged as Delta
The leading magazine and content provider for the printed packaging markets, Printing Impressions, announced its 2020
Graphic Design USA (GDUSA), a magazine and information resource for graphic design professionals, announced that Evo
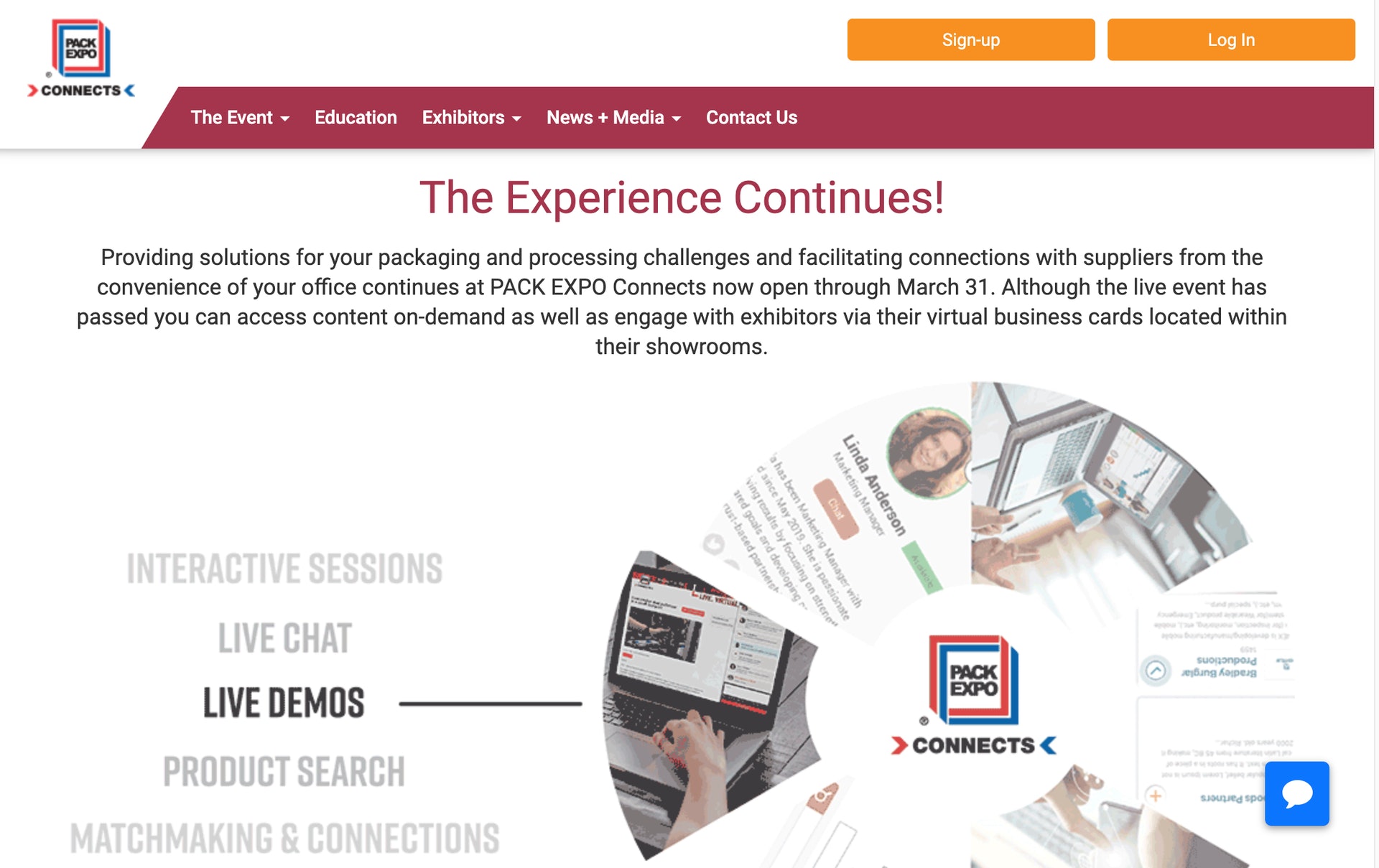
PACK EXPO Connects, produced by PMMI Media Group, debuts the most comprehensive virtual packaging event of 2020 today, providing solutions to the industry’s most critical challenges. Amazon keynotes PACK EXPO Connects at 8:30 a.m. CT with a Sunrise Breakfast Kickoff with Dr. Kim Houchens, director, customer packaging experience. Her message focuses on “Engineering your packaging to meet the challenges of eCommerce fulfillment” and includes a behind the scenes video tour of Amazon’s packaging operations.
The presentation comes on the heels of the release of PMMI Business Intelligence’s 2020 State of the Packaging Industry Report, which shows the industry as a whole on a steady path of nearly four percent growth. Further, at a recent media event PMMI Vice President of Market Development, Jorge Izquierdo, highlighted an explosive 27 percent growth of e-commerce since COVID-19 altered the retail landscape in March.
To meet these evolving needs, PACK EXPO Connects offers nearly 2,700 live product demos from more than 700 solutions-providers on an intuitive, interactive platform built for live engagement, including demos during international time zones over the course of the week.
As with all PACK EXPO events, a first-rate educational program features sessions from leading suppliers and industry experts covering key packaging trends. All content will be available both as scheduled live and on demand. Innovation Stage sessions will run noon through 2:30 p.m. CT each day through Thursday, Nov. 12. Warm up each morning at 9 a.m. CT with PACK EXPO Connects Jumpstart covering topics such as sustainability, workforce development and robotics, in English and Spanish. Presenters include Bumble Bee Foods, General Mills, L’Oréal and many more.
The show wraps with The Solution Room, only available on Friday, Nov. 13, providing a unique opportunity to collaborate with industry thought leaders from the OpX Leadership Network, Organization for Machine Automation and Control (OMAC), the Institute of Packaging Professionals and the Contract Packaging Association.
Additional learning opportunities include:
PACK EXPO Connects Trend Chats begin at 2:30 p.m. CT, Monday – Thursday. The first will feature Healthcare Packaging Editor Keren Sookne and PMMI Media Group’s VP of Content Jim Chrzan chatting with Greg Flickinger of Green Thumb International (GTI) about the industry’s response to cannabis packaging. Other chats will cover Food Processing, Track and Trace for Pharma and Food and Digital Printing.
PMMI Media Group editors will share their daily highlights and most noteworthy products and innovations Monday – Thursday at 3 p.m. CT and Friday at 2 p.m. CT via the Daily Download.
Six highlights among PACK EXPO Connects many features:
The Showcase of Packaging Innovations, sponsored by Klöckner Pentaplast and association sponsor Institute of Packaging Professionals, features winning entries from worldwide packaging competitions.
PACK EXPO Green highlights exhibitors with sustainable solutions and education sessions that promote a sustainable future.
PMMI’s Packaging & Processing Hall of Fame Class of 2020, celebrates professionals who have advanced packaging and processing, and dedicated themselves to the industry through expanding knowledge and volunteer leadership.
PMMI Showroom showcases all of the ways PMMI serves as a resource for the industry.
PACK gives BACK Miles to McCormick Walk/Run Challenge gets attendees moving and competing for prizes. Donations to the PMMI Foundation provide financial support for packaging and processing education throughout the U.S and Canada.
PubTrivia at 4 p.m. CT, supported by the Emerging Leaders Network and sponsored by Duravant, provides time for free fun, games and networking.
For more information on how to make the most of your PACK EXPO Connects experience, visit the Attendee Resource Center. This is one week to engage directly with leading packaging suppliers, see live demos in action and find the latest packaging solutions. To register visit packexpoconnects.com.
Shana Marais
Senior Manager, PR, PMMI
571.612.3184
smarais@pmmi.org

At a time when a global pandemic constrains the world, PACK EXPO Connects is bringing the industry together in record numbers this week with over 17,000 attendees as of Wednesday and more registering every day. Over 700 committed exhibitors are driving the event, taking advantage of every aspect of a completely new interactive platform.
“We are very happy with the participation in this new event. Our top priority is to connect the industry, especially in a year when professionals are unable to attend physical trade events,” says Jim Pittas, president and CEO, PMMI. “As can happen with new technology, PACK EXPO Connects faced some technical issues on Monday that delayed demos from happening as planned. But our exhibitors were resolute and remarkable, finding solutions and delivering demos despite the platform issues entirely outside of their control.”
Spee-Dee Packaging Machinery serves as a prime example of an exhibitor who pivoted on the fly to mine substantial success from PACK EXPO Connects.
“Spee-Dee was fortunate to have a successful demo on Monday via the PACK EXPO Connects platform, and then we jumped over to Facebook Live for our remaining demos,” says Dave Navin, president and CEO, Spee-Dee. “Our plan is to use the recorded content posted on the PACK EXPO Connects platform as a way to continue to build our brand and engage our customers. We have all found new ways to connect with our customers, and this provides one more tool for us.”
So far this week, PACK EXPO Connects has seen over 115,000 directory visits as of Nov. 11 with 108,000 visits to exhibitor showrooms. These interactions between solutions seekers and solution providers have led to 50,000 exhibitor leads this week alone and a total of over 110,000 leads since the web site launch driving industry innovation into 2021.
“I have found everything I have attended at PACK EXPO Connects, from exhibitor demos to speaker presentations, to be valuable experiences,” says Randy Quick, engineering manager, Kikkoman Foods, Inc. “Though not quite the same as an in-person event, the show has served as an effective virtual alternative. Educational sessions, in particular, were above average compared to other events I've seen in this format.”
In addition to demos, educational offerings – many debuting for the first time at PACK EXPO Connects – continue to produce engagement surpassing expectations. More than 800 attendees tuned in live to Dr. Kim Houchens, director, Customer Packaging Experience at Amazon’s Monday morning keynote “Engineering Your Packaging to Meet the Challenges of E-Commerce Fulfillment.” The keynote, along with The Daily Jumpstart sessions with industry experts from earlier this week, are available on demand.
The first three days of live Jumpstarts each averaged nearly 500 viewers seeking insights into key trends and technologies. And every day, over a dozen Innovation Stages and afternoon Trend Chats and Daily Downloads with PMMI Media Group editors are attracting high participation among attendees looking for insight to bring back to their plant floors.
Thursday and Friday bring two more days of hundreds of planned demos, exhibitor interactions and education. On Thursday the Innovation Stage sessions continue from 12 to 2:30 p.m. CT. The inaugural Solutions Room sessions present unique opportunities on Friday (10 a.m. to 2 p.m. CT) to foster collaboration with industry thought leaders on today’s packaging challenges with experts from the OpX Leadership Network, OMAC - Organization for Machine Automation and Control, IoPP - Institute of Packaging Professionals and the Contract Packaging Association.
For more information on how to make the most of your PACK EXPO Connects experience, visit the Attendee Resource Center. For free registration, visit packexpoconnects.com. Exhibitors will be staffing their showrooms for 1:1 chats 10 a.m. to 3 p.m. CT Thursday and 10 a.m. to 1 p.m. CT Friday. Log in to take advantage of the live interaction before the show ends on Friday.
Shana Marais
Senior Manager, PR, PMMI
571.612.3184
smarais@pmmi.org

PACK EXPO Connects brought the industry together during its launch week Nov. 9-13, with nearly 18,000 attendees engaging with more than 700 exhibitors to find solutions to critical packaging challenges.
Despite exhibitor preparedness, technical issues kept the live demonstrations from launching as intended on Monday, but the PACK EXPO Connects exhibitors adapted and demos returned for attendees looking to see technology in action the remainder of the event.
“This event was able to connect the industry, which was our number one goal in a year when professionals cannot attend physical trade events,” says Jim Pittas, president and CEO, PMMI. “And our exhibitors were remarkable in their ability to react and deliver effective demonstrations despite the technical challenges entirely outside of their control.”
To date, PACK EXPO Connects has seen 137,000 unique directory visits with 475,000 total showroom visits since the event website went live. During the show week, attendees viewed demos over 32,000 times. Those numbers will continue to grow as engagement continues on packexpoconnects.com with on-demand demos and exhibitor showrooms available through March 2021.
Exhibitor Engage Technologies Corporation and its family of companies, including Squid Ink, Eastey and AFM, attained nearly 1,000 new contacts through PACK EXPO Connects, according to Marketing Manager Josh Nelson.
“We were successful in providing live demonstrations of our equipment after switching over to the Zoom platform after [a few] technical difficulties on Monday morning,” Nelson said. “We formed a plan as a team to put our demonstration agenda into an easily emailable format and each morning I downloaded our list of demonstration attendees from the Exhibitor Section of the PACK EXPO Connects website.”
Nelson was pleased with the feedback he received regarding their virtual showroom. Customers were particularly pleased with the interactions and detailed information his team could provide in the chat as the demonstrations were live.
Josh Becker, senior manager, packaging systems, Hershey, appreciated the flexibility that the online format offered him and his team.
“At PACK EXPO Connects, I enjoyed taking advantage of attending the exhibitor demonstrations the most. It’s been helpful to attend the various sessions available and learn more about the latest cutting-edge trends in packaging operations, such as augmented reality,” Becker says. “The structure and flexibility of the show allowed us to see a diverse range of what the industry is offering.”
In addition to demos, educational offerings – many debuting for the first time at PACK EXPO Connects – produced engagement surpassing expectations. More than 800 attendees tuned in live to Dr. Kim Houchens, director, Customer Packaging Experience, at Amazon’s keynote address, which remains available on-demand at packexpoconnects.com. The daily Jumpstart sessions each averaged 400 viewers seeking insights into key trends and technologies. And every day, over a dozen Innovation Stages and afternoon Trend Chats and Daily Downloads with PMMI Media Group editors attracted high daily participation. The Solutions Room wrapped up the week on Friday with targeted interactions from the OpX Leadership Network, Organization for Machine Automation and Control (OMAC), the Institute of Packaging Professionals and the Contract Packaging Association.
“PACK EXPO Connects offered a unique opportunity to report on industry developments via a new digital format,” comments Jim Chrzan, vice president, content and brand development, PMMI Media Group. “I’m incredibly proud of our team and grateful to Amazon, General Mills, Bush Brothers and many industry experts who presented. Engagement from the packaging and processing community was exciting to see and the experience has expanded our vision of the many ways we can deliver content.”
Engagement will continue through March 31, 2021, with on-demand demos, educational sessions and showrooms remaining available as resources for industry professionals in the coming months. Access all live recordings from the show through March 2021 here.
Shana Marais
Senior Manager, PR, PMMI
571.612.3184
smarais@pmmi.org
Leading Japanese beverage player Sangaria has been counting on Sidel as a strong partner for more than nine years. To increase its production flexibility, the company once again turned to its reliable supplier by acquiring the Versatile Sidel Aseptic Combi Predis™ to handle aseptic carbonated and still drinks in PET bottles on the same line. This investment will also support Sangaria to widen its production portfolio in the future.
In Japan, the local, fast-ageing consumer base is becoming increasingly health-conscious. For food and beverage producers, this puts an increasing focus on the introduction of new, added-value products and demands a careful tailoring of the existing ones to tackle specific needs and expectations around health and packaging functionalities. Alongside, premiumisation is also projected to show strong development in this market.
According to Global Data 2020, enhanced and flavoured water, as well as tea and energy drinks, are the best performing beverages in Japan with substantial forecasted growth in the upcoming years. Seasonality, as a key purchasing factor, plays an important role for these beverages, as sparkling water is particularly preferred in summer, but green tea and coffee are more popular in the winter season. Therefore, Sangaria is continuously innovating to diversify its portfolio of drinks, which includes many soft drink brands infused with vitamins and marketed for their health benefits. The company’s motto ‘Sangaria, always searching for natural beverages’ perfectly summarises this business strategy.
Complete trust in Sidel when aseptic matters
Initially, Sangaria was using hot-fill technologies to manufacture its sensitive beverages like green teas and juices. When the company decided to increase sales with milk-based products in 2011, moving to aseptic PET production was as a better choice. For this strategic step, Sangaria turned to Sidel. Sangaria’s CEO, Ishiyama san, was personally involved in the decision and visited different Sidel customers active in aseptic beverage manufacturing and quickly understood the advantage of the safe and simple dry preform sterilisation technology from Sidel, whose reliability had already been proven worldwide.
Sangaria was the first bottling company in Japan to acquire the Aseptic Combi Predis, the Sidel integrated aseptic blow-fill-seal solution including the dry preform sterilisation. This
demonstrated the company’s pioneering spirit by exploring innovative technologies to differentiate itself from the competition and remain ahead of the market.
Great flexibility for today’s and tomorrow’s market needs
The main objective for Sangaria was to invest in a new aseptic PET packaging line, able to produce sparkling water, carbonated soft drinks (CSD) and still beverages while offering production flexibility for new product launches in the future. As carbonated beverages, including sparkling water and green tea, are booming in Japan, Sangaria was looking for an increase in its production capacity. With the Sidel Versatile Aseptic Combi Predis, they enlarged their capability to produce still beverages and CSD aseptically in PET bottles on the same line. This allows increased production flexibility with the same filling magnetic valve to aseptically handle all sensitive low and high acid products with no need for valve changeovers while ensuring reliability and product integrity. Sangaria now can manage various milk-based products and barley green tea – previously bottled on hot-filling lines – as well as healthy sparkling water in 500 ml and 1 L containers. With the new filling line, Sangaria is running the production of 500 ml bottles at 24,000 bottles per hour (bph). The production flexibility is also enhanced with the toolless mould changeover, the Bottle Switch™, which enables very fast interventions.
Safe and simple, sustainable and cost-effective aseptic production
The proven, safe and simple technology at the core of the Sidel Versatile Aseptic Combi Predis merges dry preform sterilisation with aseptic blowing, filling and sealing functions within a single production enclosure. As such, it respects the fundamental concept that underpins state-of-the-art aseptic packaging rules: producing a commercially sterile product, filled in a sterile zone, in a previously sterilised package. It differs from traditional aseptic technology because the package sterilisation takes place at the preform rather than at the bottle phase. It offers a sterilised blowing process as well as fast and safe product and format changeovers with limited manual intervention for continuous aseptic production time up to 200 hours. Plus, the producer can lower its Total Cost of Ownership (TCO) as the Versatile Aseptic Combi Predis does not use any water and very few chemicals (less than 0.7 L of H2O2 per hour) for preform sterilisation.
The sparkling drinks are produced with a Sidel carbonator that adds carbon dioxide (CO2) after the beverage processing phase. With this integrated carbonation just before filling, the
dosing performance is optimised, allowing more accuracy, reduced product waste and improved beverage stability. Moreover, it ensures one unique aseptic treatment for both still
and carbonated products.
Increase high brand recognition with lightweighted PET bottles up to 30%
Sidel not only provided the Versatile Aseptic Combi Predis, but they also partnered with
Sangaria for the PET bottle designs of their products, leveraging the 40 year experience the company has in packaging. The design of the bottle is an integral part of the brand experience, from exploiting the brand values to ensuring the all-important safety of the product up to the level of consumers’ satisfaction in using the product. Sangaria wanted to keep the same brand identity critical for recognition purposes, therefore, Sidel supported them in adapting the bottles they were handling in hot-fill to aseptic production. Thanks to the dry preform sterilisation solution they could also lightweight some of their bottles up to 30%, as this system does not require any thermal treatment of the blown bottle.
Skilled people for fast production performance
The cooperation between Sangaria and Sidel has been further continued to successfully manage the production. The operators’ expertise improved through various dedicated programs: Hazard Analysis and Critical Control Points (HACCP) and aseptic maintenance – to name but a few – were among the topics. In this way, Sangaria could achieve production targets safely and quickly. Sangaria operators and Sidel technical engineers proactively worked together during the installation and ramp-up phase at the Japanese plant.
Editors Note: The images within this document are for illustrative purposes only and should not be used for reproduction. If high resolution copies are not attached with the document, please contact Elina Kresa at F&H Porter Novelli for copies – see contact details below.
-----------------------------------
For editorial, advertising and sponsorship enquiries, please contact:
F&H Porter Novelli
Elina Kresa, Consultant
Tel: +49 (0) 89 12175-147
Email: sidel@fundh.de

It is ideal for customers that require higher capacity output than our impulse vacuum sealers.
Mostly such FIBC Jumbo Bags are used for packaging of various Chemicals, Fertilizers, Food Products, Grains, Mining P
AD*STAR® is the renowned block bottom sack made without adhesives from coated polypropylene fabric – patented worldwi
The annual Packaging Awards 2020 is one of the most recognized national competitions for industrial products.
Tell us a little bit about your background, and about your initial impression of the world of reusable plastic packag
In which processes do you use the Distribution pallet?
Our efficient tracking and automation solutions save our clients time, money, and labor.
Fully customizable
Based on the “IGO” nomadic concept thought by Shiseido, our Aptar Oyonnax team, specialized in Custom development, de
Pepsi Cola, Perrier, San Pellegrino, Coca-Cola, Heineken Beer, Sangemini, Damm SA Group, San Miguel, Schweppes, Fulle
It is constantly focused on providing new solutions, taking care of every technical and performance detail.
We decided to launch the new Online FAT service to meet customers’ requirements in this particular circumstance, havi